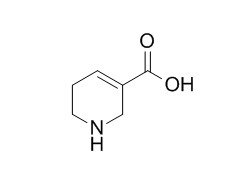
Guvacine
Guvacine can enhance the inhibition of spinal neurones by GABA.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Nanomedicine.2022, 17:6513-6525.
FASEB J.2019, 33(8):9685-9694
Konkuk University2023, 29:4634721
Cell Physiol Biochem.2019, 52(6):1255-1266
Front Chem.2024, 12:1385844.
Phytomedicine.2024, 155760.
Cell Prolif.2021, 54(8):e13083.
Food Chem.2023, 404(Pt A):134517.
Buildings2023, 13(5), 1112.
Phytomedicine.2023, 116:154841.
Related and Featured Products
Brain Research, 1977, 136(3):513-522.
Effects of the Areca nut constituents arecaidine and guvacine on the action of GABA in the cat central nervous system.[Reference:
WebLink]
METHODS AND RESULTS:
Arecaidine and Guvacine, constituents of the nut of Areca catechu, inhibited the uptake of GABA and β-alanine, but not that of glycine, by slices of cat spinal cord. In cats anaesthetised with pentobarbitone, electrophoretic arecaidine enhanced the inhibitory actions of GABA and β-alanine, but not those of glycine or taurine, on the firing of spinal neurones. Similarly, electrophoretic
Guvacine enhanced the inhibition of spinal neurones by GABA but not that by glycine. The uptake of GABA by slices of cat cerebellum was inhibited by arecaidine, and the effect of electrophoretic GABA on the firing of cerebellar Purkinje cells was enhanced by electrophoretic arecaidine.When administered intravenously arecaidine failed to affect synaptic inhibitions considered to be mediated by GABA. Intravenous arecaidine had no effect on either spinal prolonged (presynaptic) inhibition (20 mg/kg), dorsal root potentials (20 mg/kg) or basket cell inhibition of Purkinje cells (250 mg/kg), although topical arecaidine (6.6–10 × 10−3M) blocked this latter inhibition.
CONCLUSIONS:
Large doses of arecaidine (1 g/kg subcutaneous) marginally reduced the lethal effects of bicuculline in mice but appeared to have little or no anticonvulsant activity.
ChemMedChem. 2019 Jun 18;14(12):1135-1151.
Synthesis and Biological Evaluation of Nipecotic Acid and Guvacine Derived 1,3-Disubstituted Allenes as Inhibitors of Murine GABA Transporter mGAT1.[Pubmed:
30957949]
A new class of nipecotic acid and Guvacine derivatives has been synthesized and characterized for their inhibitory potency at mGAT1-4 and binding affinity for mGAT1.
METHODS AND RESULTS:
Compounds of the described class are defined by a four-carbon-atom allenyl spacer connecting the nitrogen atom of the nipecotic acid or Guvacine head with an aromatic residue. Among the compounds investigated, the mixture of nipecotic acid derivatives rac-{(Ra )-1-[4-([1,1':2',1''-terphenyl]-2-yl)buta-2,3-dien-1-yl](3R)-piperidine-3-carboxylic acid} and rac-{(Sa )-1-[4-([1,1':2',1''-terphenyl]-2-yl)buta-2,3-dien-1-yl](3R)-piperidine-3-carboxylic acid} (21 p), possessing an o-terphenyl residue, was identified as highly selective and the most potent mGAT1 inhibitor in this study. For the (R)-nipecotic acid derived form of 21 p, the inhibitory potency in [3 H]GABA uptake assays was determined as pIC50 =6.78±0.08, and the binding affinity in MS Binding Assays as pKi =7.10±0.12.
CONCLUSIONS:
The synthesis of the designed compounds was carried out by a two-step procedure, generating the allene moiety via allenylation of terminal alkynes which allows broad variation of the terminal phenyl and biphenyl subunit.