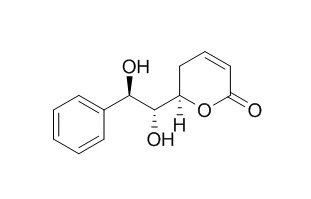
Goniodiol
(+)-Goniodiol and 7-epi-(+)-Goniodiol show high antibacterial activity (MIC, 3.1 mM) against Yersinia intermedia.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
LWT2020, 130:109535
FEBS J.2022, 10.1111:febs.16676.
National Academy Science Letters2023, s40009.
Biochem Biophys Res Commun.2021, 534:802-807.
Int J Mol Sci.2024, 25(2):764.
JAOCS2021, 98(7):779-794.
Biomolecules.2019, 9(11):E696
Biomed Pharmacother.2024, 181:117647.
Environ Toxicol.2021, 36(9):1848-1856.
Planta Med.2022, 88(9-10):794-804.
Related and Featured Products
Biosci Biotechnol Biochem. 2008 Sep;72(9):2342-52.
Syntheses of all stereoisomers of goniodiol from yeast-reduction products and their antimicrobiological activity.[Pubmed:
18776681]
All stereoisomers of Goniodiol were synthesized from yeast-reduction products. The C-6 chiral centers were converted from the chiral centers of the yeast-reduction products.
METHODS AND RESULTS:
Stereoselective conversion of the alkene, which had been prepared from the yeast-reduction product, to glycol constructed the C-7 and C-8 stereochemistry. (+)-Goniodiol and 7-epi-(+)-Goniodiol showed the highest antibacterial activity (MIC, 3.1 mM) against Yersinia intermedia.
Org Biomol Chem. 2006 May 7;4(9):1698-706.
A highly enantioselective total synthesis of (+)-goniodiol.[Pubmed:
16633562]
METHODS AND RESULTS:
A high-yielding enantioselective total synthesis of the bioactive styryllactone (+)-Goniodiol has been realised, starting from readily available (S)-glycidol. A key step is an oxygen-to-carbon rearrangement of a silyl enol ether linked via an anomeric centre, facilitating the rapid and diastereoselective construction of this functionalised system.
J Org Chem. 2002 Oct 18;67(21):7547-50.
Stereoselective syntheses of (+)-goniodiol, (-)-8-epigoniodiol, and (+)-9-deoxygoniopypyrone via alkoxyallylboration and ring-closing metathesis.[Pubmed:
12375995]
METHODS AND RESULTS:
A convenient synthesis of (+)-Goniodiol, (-)-8-epiGoniodiol, and (+)-9-deoxygoniopypyrone has been developed via asymmetric alkoxyallylboration and ring-closing metathesis pathways.