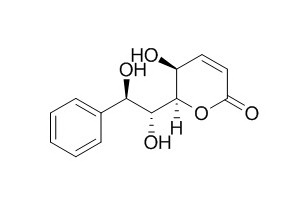
Goniotriol
Goniotriol shows antimycobacterial activity against Mycobacterium tuberculosis (MIC =100 microg/mL). It exhibits antiplasmodial activity against Plasmodium falciparum (IC50=2.6 microg/mL). Goniotriol shows cytotoxicity against cancer cells, KB, BC1, NCI-H187, and MCF-7 with IC(50) ranging from 0.4 to 22.7 microg/mL.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
World J Microbiol Biotechnol.2024, 40(9):265.
Pharmaceutics.2021, 13(7):1028.
J Sep Sci.2020, 201901140
Phytomedicine.2022, 110:154597.
Trop J Pharm Res.2023, 22(3):283-288.
Antioxidants (Basel).2021, 10(11): 1802.
Inflammation.2015, 38(4):1502-16
Cell Death Differ.2021, 1-8.
Front Pharmacol.2021, 12:744624.
Korean J of Food Science&Technology 2017, 49(2):146-150
Related and Featured Products
J Ethnopharmacol. 2009 Aug 17;125(1):47-50.
Bioactive styryllactones and alkaloid from flowers of Goniothalamus laoticus.[Pubmed:
19573585 ]
METHODS AND RESULTS:
Ten compounds, cinnamic acid (1); dihydrochrysine (2); beta-sitosterol (3); six styryllactones, (+)-3-acetylaltholactone (4), Goniotriol (5), (+)-altholactone (6), (+)-goniofufurone (7), 9-deoxygoniopypyrone (8), howiinin A (9); and an aporphine alkaloid; (-)-nordicentrine (10) were isolated from flowers of Goniothalamus laoticus. Among these, compounds 1, 3-5, 8-10 are first isolated from the Goniothalamus laoticus. Besides, compound 10 is the first report from the Goniothalamus genus. The isolated compounds were evaluated in antiplasmodial, antimycobacterial and anticancer cell lines tests.
CONCLUSIONS:
Compounds 4-6 and 10 exhibited antiplasmodial activity against Plasmodium falciparum (IC(50) 2.6, 7.9, 2.6 and 0.3 microg/mL, respectively), while 5, 6, 9 and 10 showed antimycobacterial activity against Mycobacterium tuberculosis (MIC 100, 6.25, 6.25 and 12.5 microg/mL, respectively). In addition, compounds 4-10 showed cytotoxicity against cancer cells, KB, BC1, NCI-H187, and MCF-7 with IC(50) ranging from 0.4 to 22.7 microg/mL.
J Org Chem. 2008 Jan 4;73(1):2-11.
Stereoselective total synthesis of bioactive styryllactones (+)-goniofufurone, (+)7-epi-goniofufurone, (+)-goniopypyrone, (+)-goniotriol, (+)-altholactone, and (-)-etharvensin.[Pubmed:
17523660]
METHODS AND RESULTS:
Stereoselective total synthesis of biologically active styryllactones 7-epi-goniofufurone, goniofufurone, goniopypyrone, Goniotriol, altholactone, and etharvensin was achieved in high overall yields from a common intermediate derived from d-(-)-tartaric acid. It is based on the utility of a masked tetrol, comprising an alkene tether and four contiguous hydroxy groups. The pivotal reaction sequence involves hydroxy-directed lactonization via the oxidation of alkene, and subsequent elaboration to styryllactones.
CONCLUSIONS:
The masked tetrol was prepared by the extension of gamma-phenyl-gamma-hydroxy butyramide, readily obtained from the bis-dimethylamide of tartaric acid, employing a combination of selective Grignard additions and a stereoselective reduction.
J Nat Prod. 1989 Nov-Dec;52(6):1371-3.
Goniotriol from Goniothalamus giganteus.[Pubmed:
2614426]
METHODS AND RESULTS:
The known styrylpyrone, Goniotriol, has been isolated from Goniothalamus giganteus. Its bioactivities are reported, and its structure and relative stereochemistry have been determined by X-ray crystallography as 6R-(7R,8R-dihydro-7,8-dihydroxystyryl)-5S,6R-dihydro-5-hydroxy-2-p yrone.
Obovatol
Catalog No: CFN95054
CAS No: 83864-78-2
Price: $318/5mg
Oleuroside
Catalog No: CFN95099
CAS No: 116383-31-4
Price: $288/20mg
7-(4-Hydroxyphenyl)-1-phenyl-4-hepten-3-one
Catalog No: CFN95140
CAS No: 100667-52-5
Price: $318/5mg
3,7,25-Trihydroxycucurbita-5,23-dien-19-al
Catalog No: CFN95150
CAS No: 85372-65-2
Price: $318/5mg
(3R,5S,E)-1,7-Diphenylhept-1-ene-3,5-diol
Catalog No: CFN95227
CAS No: 232261-31-3
Price: $318/5mg
N-trans-p-Coumaroyltyrosine
Catalog No: CFN95279
CAS No: 77201-66-2
Price: $318/10mg
Toddalolactone 3'-O-methyl ether (6-(2-Hydroxy-3-methoxy-3-methylbutyl)-5,7-dimethoxycoumarin)
Catalog No: CFN95372
CAS No: 143614-35-1
Price: $318/10mg
Bocconoline
Catalog No: CFN95373
CAS No: 32906-88-0
Price: $413/5mg
2,6-Dihydroxyacetophenone-4-O-[4',6'-(S)-hexahydroxydiphenoyl]-beta-D-glucose
Catalog No: CFN95470
CAS No: 1781226-44-5
Price: $318/5mg
New compound 21
Catalog No: CFN95560
CAS No: N/A
Price: $413/5mg