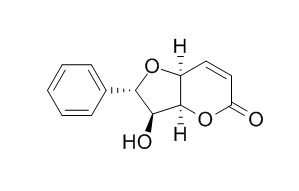
Isoaltholactone
Standard reference
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biomol Ther (Seoul).2023, 31(1):40-47.
Chung Shan Medical University2020, US20200323790A1
Nutrients.2024, 16(15):2518.
Cell Physiol Biochem.2017, 44(4):1381-1395
Environ Toxicol.2020, doi: 10.1002
J Liq Chromatogr R T2025, 2505536.
Food Chem. 2020, 320:126530
Plant Sci.2021, 313:111069.
Heliyon2020, 6(6):e04337.
J Exp Bot.2016, 67(12):3777-88
Related and Featured Products
Tetrahedron Letters.2003 July 28;44(31):5831–5833.
A concise and stereoselective synthesis of both enantiomers of altholactone and isoaltholactone.[Reference:
WebLink]
METHODS AND RESULTS:
A concise and flexible stereoselective route to synthesize both enantiomers of the highly functionalized α,β-unsaturated-δ-lactones, altholactone and Isoaltholactone, from readily available cinnamyl alcohol is described. This approach derived its asymmetry from Sharpless catalytic asymmetric epoxidation and Sharpless asymmetric dihydroxylation reactions.
CONCLUSIONS:
The resulting diols were produced in high enantiomeric excess and were cyclized in a stereoselective manner in the presence of a catalytic amount of camphor sulphonic acid.
European Journal of Organic Chemistry. 2011 Dec; 2011(36).
Stereoselective Synthesis of Aza Analogues of Isoaltholactone and Goniothalesdiol - New Applications of the Heck-Matsuda Reaction.[Reference:
WebLink]
The stereoselective synthesis of nitrogen analogues of biologically active Isoaltholactone and goniothalesdiol are described.
METHODS AND RESULTS:
The successful strategy employed a Heck–Matsuda reaction between a chiral endocyclic enecarbamate bearing an ester functionality and arenediazonium tetrafluoroborates in a divergent approach at an early stage of the synthesis. Several aspects related to this critical arylation reaction are discussed to highlight structural features that affect the outcome of the arylation process. The synthesis of (–)-aza-Isoaltholactone 6 was successfully accomplished in nine steps from the starting enecarbamate.
We also performed the synthesis of the new fully substituted pyrrolidine (–)-(2R,3R,4S,5S)-1-(tert-butoxycarbonyl)-3,4-dihydroxy-5-phenylpyrrolidin-2-ylacrylic acid 28, which is a potential advanced intermediate in the route to aza-altholactone.
Moreover, the synthesis of a new nitrogen analogue of goniothalesdiol (+)-33 was accomplished from the protected dihydroxypyrrolidine (–)-27, obtained from an attempted synthesis of aza-altholactone.