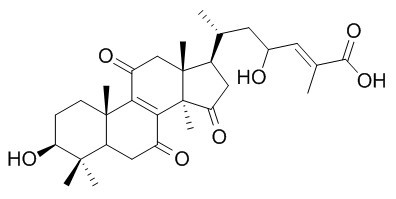
Ganoderic acid Z
The binding affinities of ganoderic acid DM and ganoderic acid Z (ΔGbind, −16.83 and−10.99 kcal mol−1) are comparable to that of current commercial drug oseltamivir (−23.62 kcal mol−1);Ganoderic acid DM is a potential source of anti-influenza ingredient, with novel binding pattern and advantage over oseltamivir, it has steric hindrance on the 150 cavity of N1 protein, and exerts activities across the H274Y and N294S mutations, is the attractive candidates of novel neuraminidase (NA) inhibitors.Ganoderic acid zeta has cytotoxicity in vitro against Meth-A and LLC cell lines.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
BMC Complement Altern Med.2018, 18(1):221
J Ethnopharmacol.2017, 198:91-97
Fitoterapia.2022, 157:105130.
J Neuroinflammation.2023, 20(1):268.
Genes Genomics.2020, 10.1007
Microb Biotechnol.2021, 14(5):2009-2024.
Evid Based Complement Alternat Med.2018, 2018:8565132
Front Microbiol.2023, 14:1232039.
Herbal Formula Science2024, 32(3):203-221
Anal Bioanal Chem.2016, 408(1):177-90.
Related and Featured Products
Chem Pharm Bull (Tokyo). 2000 Jul;48(7):1026-33.
Triterpenes from the spores of Ganoderma lucidum and their cytotoxicity against meth-A and LLC tumor cells.[Pubmed:
10923835]
METHODS AND RESULTS:
Six new highly oxygenated lanostane-type triterpenes, called ganoderic acid gamma (1), ganoderic acid delta (2), ganoderic acid epsilon (3), Ganoderic acid Zeta (Ganoderic acid Z,4), ganoderic acid eta (5) and ganoderic acid theta (6), were isolated from the spores of Ganoderma lucidum, together with known ganolucidic acid D (7) and ganoderic acid C2 (8). Their structures of the new triterpenes were determined as (23S)-7beta,15alpha,23-trihydroxy-3,11-dioxolanosta-8, 24(E)-diene-26-oic acid (1), (23S)-7alpha,15alpha23-trihydroxy-3,11-dioxolanosta-8, 24(E)-diene-26-oic acid (2), (23S)-3beta3,7beta, 23-trihydroxy-11,15-dioxolanosta-8,24(E)-diene-26-oic acid (3), (23S)-3beta,23-dihydroxy-7,11,15-trioxolanosta-8, 24(E)-diene-26-oic acid (4), (23S)-3beta,7beta,12beta,23-tetrahydroxy-11,15-dioxolanos ta-8,24(E)-diene-26-oic acid (5) and (23S)-3beta,12beta23-trihydroxy-7,11,15-trioxolanosta-8,24(E )-diene-26-oic acid (6), respectively, by chemical and spectroscopic means, which included the determination of a chiral center in the side chain by a modification of Mosher's method. The cytotoxicity of the compounds isolated from the Ganoderma spores was carried out in vitro against Meth-A and LLC tumor cell lines.
J. Mol .Graph. Model, 2016, 65:27-34.
Novel binding patterns between ganoderic acids and neuraminidase: Insights from docking, molecular dynamics and MM/PBSA studies.[Pubmed:
26905206 ]
Recently, ganoderic acids (GAs) give rise to the attractive candidates of novel neuraminidase (NA) inhibitors. However, there is still no evident conclusion about their binding patterns.
METHODS AND RESULTS:
To this end, docking, molecular dynamics and MM/PBSA methods were combined to study the binding profiles of GAs with the N1 protein and familiar H274Y and N294S mutations (A/Vietnam/1203/04 stain). It was found that the binding affinities of Ganoderic acid DM and Ganoderic acid Z (ΔGbind, -16.83 and -10.99 kcal mol(-1)) are comparable to that of current commercial drug oseltamivir (-23.62 kcal mol(-1)). Electrostatic interaction is the main driving force, and should be one important factor to evaluate the binding quality and rational design of NA inhibitors. The 150-loop residues Asp151 and Arg152 played an important role in the binding processes. Further analysis revealed that ganoderic acid DM is a potential source of anti-influenza ingredient, with novel binding pattern and advantage over oseltamivir. It had steric hindrance on the 150 cavity of N1 protein, and exerted activities across the H274Y and N294S mutations. This work also pointed out how to effectively design dual-site NA inhibitors and reinforce their affinities.
CONCLUSIONS:
These findings should prove valuable for the in-depth understanding of interactions between NA and GAs, and warrant the experimental aspects to design novel anti-influenza drugs.