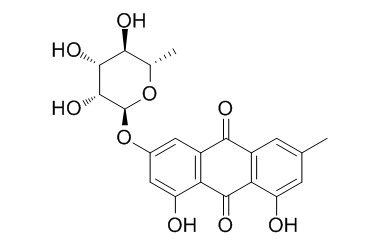
Frangulin A
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Pharmaceuticals (Basel).2021, 14(7):633.
Molecules.2023, 28(8):3291.
Front. Pharmacol.2022, 901563.
Antioxidants (Basel).2024, 13(12):1530.
Research on Crops.2017, 18(2)
International J of Green Pharmacy2019, 13(3)
J Cachexia Sarcopenia Muscle.2022, 13(6):3149-3162.
Nutrients.2019, 12(1):E40
Trop J Nat Prod Res.2019, 3(1):6-9
Int J Mol Sci.2023, 24(8):7442.
Related and Featured Products
Magnetic Resonance in Chemistry, 1998, 36(10):769-772.
Assignment of the 1H and 13C NMR spectra of anthraquinone glycosides from Rhamnus frangula.[Reference:
WebLink]
1H and 13C NMR chemical shifts are reported for five anthraquinone glycosides from Rhamnus frangula (Alder Buckthorn).
METHODS AND RESULTS:
The investigation utilized data obtained by a variety of 1D and 2D NMR techniques such as homodecoupling, NOE, J‐modulated spin‐echo, COSY, HSC and COLOC. Assignments were made for Frangulin A and frangulin B and glucoFrangulin A and B. A further compound previously thought to be a glycoside of emodin is shown to be physcion‐8‐O‐β‐D‐glucoside.
Planta Med 2013; 79 - PK33.
New HPLC – method to determine Frangulin A and B as well as Glucofrangulin A and B in Frangulae cortex.[Reference:
WebLink]
The current monograph in the European Pharmacopeia for Frangulae cortex describes a photometric assay based on an adapted bornträger reaction to determine hydroxyanthracene glycosides, calculated as frangulin B. The method is time consuming, unspecific for frangulines and the precision is not adequate for a modern assay. The photometric method shall therefore be replaced by a modern HPLC-method. There is no HPLC method published in the literature that allows the determination of Frangulin A/frangulin B and glucoFrangulin A/glucofrangulin B in Frangulae cortex.
METHODS AND RESULTS:
About 300 mg of freshly milled drug are extracted for 15 min with ultrasound. The extraction solution consists of acetonitrile/water 50:50 v/v and 2 g/L NaHCO3. A conventional RP C18 Nucleodur (4 mm x 125 mm), 3 µm was used as stationary phase. Mobile phase A consists of water (pH of 2.0, adjusted with phosphoric acid). Mobile phase B consists acetonitrile/methanol 20:80 v/v. The flow rate is 1.0 mL/min, the detection wavelength 435nm, the column temperature is 50 °C, and the injection volume 20µL. The gradient is shown in table 1.
CONCLUSIONS:
The mobile phase separates the four frangulins sufficiently. Results of several samples will be presented on the poster. A chromatogram from a Frangulae cortex sample is shown in figure 1.