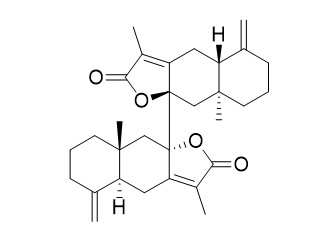
Biatractylolide
Biatractylolide has a neuroprotective effect on glutamate-induced injury in PC12 and SH-SY5Y cells through a mechanism of the PI3K-Akt-GSK3β-dependent pathways. The molecular mechanisms of inhibitory activities of biatractylolide on AChE are not only through binding to AChE, but also via reducing AChE expression by inhibiting the activity of GSK3β.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biol Pharm Bull.2021, 44(12):1891-1893.
Plant Cell Physiol.2018, 59(1):128-141
Antibiotics.2022, 11(4), 510.
Front Cell Dev Biol.2020, 8:32.
Int J Mol Sci.2024, 25(1):616.
J Microbiol Biotechnol.2020, 30(2):178-186.
J Food Biochem.2021, 45(7):e13774.
Phytomedicine.2022, 99:154025.
Food Bioscience2022, 50:102187.
Phytomedicine.2019, 67:153159
Related and Featured Products
Evid Based Complement Alternat Med. 2017;2017:1291458.
Biatractylolide Modulates PI3K-Akt-GSK3β-Dependent Pathways to Protect against Glutamate-Induced Cell Damage in PC12 and SH-SY5Y Cells.[Pubmed:
29075302]
Biatractylolide, isolated from the ethyl acetate extract of Atractylodes macrocephala, has shown various pharmacological activities such as antitumor and antioxidant activities.
CONCLUSIONS:
In this work, we aim to study the protective effect of Biatractylolide on glutamate-induced rat adrenal pheochromocytoma cell (PC12) and human bone marrow neuroblastoma cell line (SH-SY5Y) injury and preliminarily explore its mechanism. The results showed that glutamate was cytotoxic with an inhibitory concentration 50% (IC50) of 8.5 mM in PC12 and 10 mM in SH-SY5Y cells. In this work, the preincubation with Biatractylolide (10, 15, and 20 μM) observably improved cell viability, inhibited the apoptosis of cells induced by glutamate, and reduced the activity of LDH. AO staining revealed that apoptosis of cells was decreased. Additionally, the results of western blotting manifested that pretreatment with Biatractylolide could downregulate GSK3β protein expression and upregulate p-Akt protein expression, thereby protecting PC12 and SH-SY5Y cells from injury.
CONCLUSIONS:
All these findings indicate that Biatractylolide has a neuroprotective effect on glutamate-induced injury in PC12 and SH-SY5Y cells through a mechanism of the PI3K-Akt-GSK3β-dependent pathways.
Evid Based Complement Alternat Med. 2016;2016:7481323.
Primary Investigation for the Mechanism of Biatractylolide from Atractylodis Macrocephalae Rhizoma as an Acetylcholinesterase Inhibitor.[Pubmed:
27642355]
METHODS AND RESULTS:
Biatractylolide was isolated from ethyl acetate extract of dried Atractylodis Macrocephalae Rhizoma root by multistep chromatographic processing. Structure of Biatractylolide was confirmed by (1)H-NMR and (13)C-NMR. The IC50 on acetylcholinesterase (AChE) activity was 6.5458 μg/mL when the control IC50 value of huperzine A was 0.0192 μg/mL. Molecular Docking Software (MOE) was used to discover molecular sites of action between Biatractylolide and AChE protein by regular molecular docking approaches. Moreover, Biatractylolide downregulated the expression of AChE of MEF and 293T cells in a dose-dependent manner.
CONCLUSIONS:
These results demonstrated that the molecular mechanisms of inhibitory activities of Biatractylolide on AChE are not only through binding to AChE, but also via reducing AChE expression by inhibiting the activity of GSK3β.
Chem Pharm Bull (Tokyo). 2002 Jul;50(7):964-5.
The crystal structure of biatractylolide, an 8,8' (C-C) linked dimeric 12,8-eudesmanolide from the resin of Trattinickia rhoifolia WILLD.[Pubmed:
12130855 ]
METHODS AND RESULTS:
A symmetrical dimeric sesquiterpenoid, Biatractylolide (1), was isolated from the resin of Trattinickia rhoifolia WILLD.
CONCLUSIONS:
The structure of compound 1 was elucidated by one- and two-dimensional NMR techniques and electron impact-mass spectra (EI-MS) data, and confirmed by X-ray crystallographic analysis.