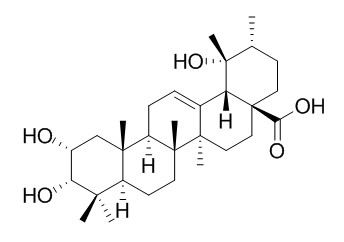
Euscaphic acid
Euscaphic acid has anti-diabetic, and anti-inflammatory activities, it
inhibits LPS-induced inflammatory responses by interference with the clustering of TRAF6 with IRAK1 and TAK1, resulting in blocking the activation of IKK and MAPKs signal transduction to downregulate NF-κB activations. Euscaphic acid induces death by activation of caspase-3, dependent apoptotic pathway. Euscaphic acid and tormentic acid have inhibitory effect on high fat diet-induced obesity in the rat. Euscaphic acid also has anti-contraction effects on rat’s aortic smooth muscle.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
HIV Med.2021, 22(8):690-704.
Planta Medica International2022, 9(01):e108-e115.
Plants (Basel).2021, 10(7):1376.
Food Chem Toxicol.2023, 176:113785.
Hum Exp Toxicol.2022, 41:9603271221143713.
J Appl Biol Chem.2022, 65(4):pp.463-469.
Heliyon.2022, e12337.
J of the Society of Cosmetic Scientists of Korea2018, 44(4):407-417
J Ethnopharmacol.2017, 209:305-316
Journal of Food Composition and Analysis2021, 100:103905.
Related and Featured Products
Adv Life Sci. Technol., 2015, 33.
Anti-Contraction Effects of Euscaphic Acid Isolated from Crataegus azarolus var. aronia L on Rat’s Aortic Smooth Muscle.[Reference:
WebLink]
The current study represents the first attempt to investigate the effect of the Euscaphic acid (EA) on Rats isolated thoracic aortic smooth muscle cells.
METHODS AND RESULTS:
Isolated aorta was used to test the anti-contraction effects and the possible mode of action(s) of the EA (1*10 -7 M) and (3*10 -7 M) isolated from Crataegus azarolus var. aronia L. Euscaphic acid showed high anti-contraction effects on norepinephrin (NE), (1*10 -9 -10 -4 M) induced contraction in aortic smooth muscle cells in endothelium-intact, endothelium-denuded, and aortic rings pre-incubated with potassium (K + )-channels blocker (tetraethylammonium, TEA), prostaglandin I 2 (PGI ) inhibitor (indomethacin) and cyclic guanosine monophosphate (cGMP) inhibitor ( methylene blue). On the other hand, other K 2 channels subtype blockers glibenclamide (GLIB); barium chloride (BaCl ) and 4-aminopyridine (4-AP) demonstrated that adenosine triphosphate sensitive K + (K ATP ), inwardly rectifying K 2 + (K ir ) and voltage-dependent K ) channels played no role in anti-contraction induced by EA. Furthermore, the role of L-types calcium (Ca ) channels in EA anti-contractile effects on aortic smooth muscle cells was proved, by using the Ca -channel blocker verapamil, as indicated by the production of a potent anti-contraction effect . The results of the current study indicate that the anti-contraction effects of EA may be due to the activation of calcium dependent, K ) channels and blocking of L-type Ca ++ channels.
CONCLUSIONS:
Thus, from these results it can be concluded that both K and Ca ++ channels play an important role in anti-contraction effects of EA, which are mediated possibly through opening of K channels and blockade of voltage-dependent calcium channels, which may justify the use of medicinal plant C. azarolus in cardiovascular disease.
Am J Transl Res . 2019 Apr 15;11(4):2090-2098. eCollection 2019.
Euscaphic acid inhibits proliferation and promotes apoptosis of nasopharyngeal carcinoma cells by silencing the PI3K/AKT/mTOR signaling pathway[Pubmed:
31105820]
Abstract
Rubus alceaefolius Poir. has been used for the treatment of nasopharyngeal carcinoma (NPC) in China for many years. Euscaphic acid is an active component of Rubus alceaefolius Poir. However, the mechanism of action of Euscaphic acid in NPC remains unclear. In this study, Euscaphic acid inhibited the proliferation of NPC cells, induced apoptosis, and led to cell cycle arrest in the G1/S phase. In addition, Euscaphic acid inhibited the expression of phosphatidylinositide 3-kinases (PI3K), phosphorylated protein kinase B (p-AKT), and phosphorylated mammalian target of rapamycin (p-mTOR) p-mTOR in NPC cells. The activation of the PI3K/AKT/mTOR signaling pathway by IGF-1 promoted cell proliferation, inhibited apoptosis, and cell cycle arrest in NPC cells. In conclusion, we demonstrated that Euscaphic acid reduced cell proliferation and induced apoptosis and cell cycle arrest in NPC cells by suppressing the PI3K/AKT/mTOR signaling pathway.
Keywords: AKT; Euscaphic acid; PI3K; mTOR; nasopharyngeal carcinoma.
Korean J. Pharmacogn., 2005, 36(4):324-31.
Inhibitory effect of euscaphic acid and tormentic acid from the roots of Rosa rugosa on high fat diet-induced obesity in the rat.[Reference:
WebLink]
The roots of Rosa rugosa have been used to treat diabetes mellitus in the folkloric society of Korea.
METHODS AND RESULTS:
To demonstrate the active component for the rat obesity induced by high fat diet for 6 weeks, the phytochemical fractionation and the pharmacological activity test were performed on this crude drug. It was shown that the methanolic extract and its EtOAc fraction inhibited the weight increase of the rat body, abdominal fat pad and hyperlipidemia at 200 mg/kg dose. Further, the triterpenoids, Euscaphic acid and tormentic acid, isolated from R. rugosa roots were active at 30 mg/kg in the same assay. The two components shifted serum total-, HDL, and LDL-cholesterol levels toward the values of the unteated group, suggesting that the active compounds has hypolipidemic effects. The rats fed Euscaphic acid and tormentic acid also reduced thiobarbituric acid-reactive substance (TBARS) and hydroxyl radical in the rat blood and increased Superoxide dismutase activity compared to the control. TBARS values and carbonyl content of the hepatic protein were reduced by treatment with the two triterpenoids. Antioxidative enzyme (SOD, glutathione peroxidase, and catalase) activities in hepatic tissues were increased by treatment of rats with the triterpenoids, which suggests that triterpenoids inhibited the reduction of hepatic antioxidative activity caused by high fat diet.
CONCLUSIONS:
Taken together, these results support that Euscaphic acid and tormentic acid improve a high fat diet-induced hyperlipidemia via the activation of antioxidative mechanism.
J Cell Biochem. 2012 Jun;113(6):1936-46.
Euscaphic acid isolated from roots of Rosa rugosa inhibits LPS-induced inflammatory responses via TLR4-mediated NF-κB inactivation in RAW 264.7 macrophages.[Pubmed:
22234926]
As an attempt to search for bioactive natural products exerting anti-inflammatory activity, we have evaluated the anti-inflammatory effects of Euscaphic acid (19α-hydroxyursane-type triterpenoids, EA) isolated from roots of Rosa rugosa and its underlying molecular mechanisms in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages.
METHODS AND RESULTS:
Euscaphic acid concentration-dependently reduced the production of nitric oxide (NO), prostaglandin E2 (PGE2), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β) induced by LPS in RAW 264.7 macgophages. Consistent with these data, expression levels of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) protein and iNOS, COX-2, TNF-α, and IL-1β mRNA were inhibited by Euscaphic acid in a concentration-dependent manner. In addition, Euscaphic acid attenuated LPS-induced DNA binding and transcriptional activity of nuclear factor-kappa B (NF-κB), which was accompanied by a parallel reduction of degradation and phosphorylation of inhibitory kappa Bα (IκBα) and consequently by decreased nuclear translocation of p65 subunit of NF-κB. Pretreatment with Euscaphic acid significantly inhibited the LPS-induced phosphorylation of IκB kinase β (IKKβ), p38, and JNK, whereas the phosphorylation of ERK1/2 was unaffected. Furthermore, Euscaphic acid interfered with the LPS-induced clustering of TNF receptor-associated factor 6 (TRAF6) with interleukin receptor associated kinase 1 (IRAK1) and transforming growth factor-β-activated kinase 1 (TAK1).
CONCLUSIONS:
Taken together, these results suggest that Euscaphic acid inhibits LPS-induced inflammatory responses by interference with the clustering of TRAF6 with IRAK1 and TAK1, resulting in blocking the activation of IKK and MAPKs signal transduction to downregulate NF-κB activations.
Pharmazie. 2008 Oct;63(10):765-7.
Euscaphic acid, a new hypoglycemic natural product from Folium Eriobotryae.[Pubmed:
18972842]
Folium Eriobotryae has been used as a medicinal plant for a long time, and it is known to have many physiological actions such as anti-inflammatory, anti-tussive, expectorant and anti-diabetic.
We have reported that the 70% ethanol extract of Folium Eriobotryae exerted a significant hypoglycemic effect to alloxan-diabetic mice.
METHODS AND RESULTS:
In this study, we isolated Euscaphic acid, a natural product from Folium Eriobotryae, and investigated its hypoglycemic effect in normoglycemic and alloxan-diabetic mice. All effects had been compared with those of gliclazide. The plasma glucose levels were significantly lowered in normoglycemic mice treated with Euscaphic acid compared to mice treated with 0.5% CMC-Na solution only. Moreover, the dosage of 50 mg/kg exerted a significant (P < 0.05) hypoglycemic effect in alloxan-diabetic mice after orally administration.
CONCLUSIONS:
The research proved that Euscaphic acid is one of the active hypoglycemic constituents in Folium Eriobotryae, but the details of the mechanism need to be investigated further.