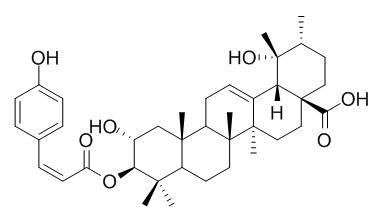
3-O-cis-p-Coumaroyltormentic acid
3beta-O-cis-p-Coumaroyltormentic acid, and 3beta-O-trans-p-coumaroyltormentic acid are weakly selective for vancomycin-resistant Enterococcus (VRE) compared with eukaryotic cells, with an MIC of 59.4microg/mL and a 50% inhibitory concentration (IC50) of 72.0microg/mL for monkey kidney epithelial (MA104) cells. A mixture of 3-O-cis-p-coumaroyltormentic acid and 3-O-trans-p-coumaroyltormentic acid shows an inhibitory effect comparable to (-)-epigallocatechin gallate (EGCG) of green tea on the activation of Epstein-Barr virus early antigen (EBV-EA) induced by 12-O-tetradeca-noylphorbol-13-acetate (TPA).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Environ Toxicol.2024, 39(4):2417-2428.
Int J Mol Sci.2020, 21(9):3144.
J Sep Sci.2020, 201901140
Phytochem Anal.2023, pca.3305.
Malaysian Journal of Analytical Sciences2023, 27(4):840-848.
Biochem Biophys Res Commun.2018, 505(1):194-200
Int J Biol Sci.2023, 19(10):3077-3098.
Molecules.2019, 24(2):329
Int Immunopharmacol.2023, 125:111175.
Food Res Int.2017, 96:40-45
Related and Featured Products
J. Ethnopharmacol.,2008 Mar 28;116(3):554- 60.
Antibacterial compounds from Planchonia careya leaf extracts.[Pubmed:
18289814 ]
METHODS AND RESULTS:
Six known compounds were isolated from the leaf extracts and were identified as 1, (+)-gallocatechin; 2, gallocatechin-(4alpha-->8)-gallocatechin; 3, 9(S)-hydroxy-10E,12Z-octadecadienoic acid (alpha-dimorphecolic acid); 4, 2alpha,3beta,24-trihydroxyolean-12-en-28-oic acid (hyptatic acid-A); 5, 3beta-O-cis-p-coumaroyltormentic acid(3-O-cis-p-Coumaroyltormentic acid ); and 6, 3beta-O-trans-p-coumaroyltormentic acid.
CONCLUSIONS:
Compounds 5 and 6 were weakly selective for vancomycin-resistant Enterococcus (VRE) compared with eukaryotic cells, with an MIC of 59.4microg/mL and a 50% inhibitory concentration (IC(50)) of 72.0microg/mL for MA104 cells.
Phytochemistry,2002 Feb;59(3):315-23.
Production of bioactive triterpenes by Eriobotrya japonica calli.[Pubmed:
11830140]
Callus tissue cultures induced from an axenic leaf of Eriobotrya japonica (Rosaceae) produced triterpenes in large amounts (ca. 50 mg/g dry wt).
METHODS AND RESULTS:
Nine triterpenes were characterized as ursolic acid, oleanolic acid, 2alpha-hydoxyursolic acid, maslinic acid, tormentic acid, 2alpha, 19alpha-dihydroxy-3-oxo-urs-12-en-28-oic acid, hyptadienic acid and a mixture of 3-O-cis-p-Coumaroyltormentic acid and 3-O-trans-p-coumaroyltormentic acid. The triterpene composition in the callus tissues was noticeably different from that in intact leaves. The contents of tormentic acid with antidiabetic action, and 2alpha, 19alpha-dihydroxy-3-oxo-urs-12-en-28-oic acid with anti-HIV activity, were much larger than those in the intact leaves.
CONCLUSIONS:
All of the triterpenes isolated from the callus tissues showed an inhibitory effect comparable to (-)-epigallocatechin gallate (EGCG) of green tea on the activation of Epstein-Barr virus early antigen (EBV-EA) induced by 12-O-tetradecanoylphorbol-13-acetate (TPA).
2alpha, 19alpha-Dihydroxy-3-oxo-urs-12-en-28-oic acid was the most potent inhibitor among them and caused a significant delay of two-stage carcinogenesis on mouse skin.