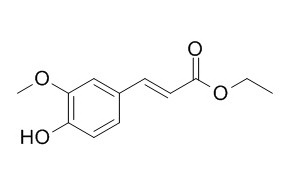
Ethyl (E)-ferulate
Ethyl ferulate could be used for therapeutic purposes as a potent inducer of HO-1 for the protection of brain cells against oxidative and neurodegenerative conditions.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Chem X.2024, 24:101909.
Biol. Life Sci. Forum2024, 35(1), 6.
Pharm Biomed Res2023, 9(3):173-182.
Appl. Sci.2020, 10(4),1304
Pharmaceutics.2020, 12(9):845.
Separations2021, 8(7),90.
Bull. Pharm. Sci., Assiut University2020, 43(2):149-155.
BMC Complement Altern Med.2019, 19(1):325
J Microbiol Biotechnol.2022, 32(2):141-148.
Food Bioscience2023, 53:102687
Related and Featured Products
Journal of Biotechnology, 2013, 164(2):340-345.
Solvent-free enzymatic transesterification of ethyl ferulate and monostearin: Optimized by response surface methodology.[Reference:
WebLink]
In the CNS, the heme oxygenase (HO) system has been reported to be active and to operate as a fundamental defensive mechanism for neurons exposed to an oxidant challenge. We have recently shown that both curcumin and caffeic acid phenethyl ester, two phenolic natural compounds, potently induce HO-1 expression and activity in rat astrocytes. We have extended our previous findings examining the effects of two other plant-derived phenolic compounds, with analogous chemical structures, in rat astrocytes and neurons.
METHODS AND RESULTS:
Ethyl ferulate (Ethyl (E)-ferulate, ethyl 4-hydroxy-3-methoxycinnamate) (EFE), the naturally occurring ester of ferulic acid, was able to induce HO-1 protein expression. Maximal expression of HO-1 mRNA and protein and a significant increase in HO activity were detected after 6 h of incubation with 15 microM EFE in astrocytes and 5 microM EFE in neurons. Higher concentrations of EFE (50 microM) caused a substantial cytotoxic effect with no change in HO-1 protein expression and activity. Exposure of astrocytes to resveratrol, a phytoalexin derived from grapes, resulted in an increase of HO-1 mRNA, but it was not able to induce HO-1 protein expression and activity. Interestingly, preincubation (12 h) of neurons with EFE resulted in an enhanced cellular resistance to glucose oxidase-mediated oxidative damage; this cytoprotective effect was considerably attenuated by zinc protoporphyrin IX, an inhibitor of HO activity.
CONCLUSIONS:
This study identifies a novel natural compound that could be used for therapeutic purposes as a potent inducer of HO-1 for the protection of brain cells against oxidative and neurodegenerative conditions.