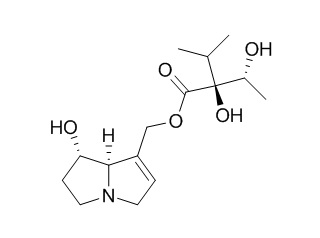
Rinderine
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Turkish Journal of Pharmaceutical Sciences2022, DOI: 10.4274
Molecules. 2013, 18(11):14105-21
J Agric Food Chem.2021, 69(14):4210-4222.
Sci Rep. 2018, 10590
Molecules2022, 27(12):3824.
J Agric Food Chem.2024, acs.jafc.4c01387.
Pharmaceutics.2023, 15(6):1771.
HIV Med.2021, 22(8):690-704.
Chinese Pharmacological Bulletin2019, 35(8):1120-1125
J of the Korean Society of Cosmetics and Cosmetology2018, 399-406
Related and Featured Products
Chemoecology 2003:13(1): 55-62
Uptake and metabolism of rinderine and retronecine in leaf-beetles of the genus Platyphora and alkaloid accumulation in the exocrine defensive secretions[Reference:
WebLink]
Sequestration and processing of pyrrolizidine alkaloids (PAs) by leaf beetles of the genus Platyphora were investigated.
METHODS AND RESULTS:
Tracer experiments with labeled alkaloids were performed with P. eucosma feeding on Koanophyllon panamense (Asteraceae, tribe Eupatorieae). P. eucosma catalyzes the same reactions previously demonstrated for P. boucardi specialized to Prestonia portobellensis (Apocynaceae): (i) epimerization of Rinderine to intermedine; (ii) esterification of retronecine yielding insect-specific PAs; (iii) efficient transport of the PAs as free bases into the defensive secretions. P. bella feeding on Tournefortia cuspidata (Boraginaceae) shows the same sequestration behavior and ability to synthesize the specific retronecine esters.
CONCLUSIONS:
P. ligata, a species phylogenetically closely related to the PA adapted species and clustering in the same clade, but feeding on a host plant devoid of PAs, feeds easily on PA treated host-plant leaves, but does not sequester or metabolize PAs. P. kollari a species clustering outside the PA clade refused to feed on its food-plant leaves painted with PAs. The results are discussed in relation to host-plant selection of the PA adapted species and the role of PAs in chemical defense.
Phytochem Anal. 2014 Sep-Oct;25(5):429-38.
Semi-automated separation of the epimeric dehydropyrrolizidine alkaloids lycopsamine and intermedine: preparation of their N-oxides and NMR comparison with diastereoisomeric rinderine and echinatine.[Pubmed:
24816769]
The diversity of structure and, particularly, stereochemical variation of the dehydropyrrolizidine alkaloids can present challenges for analysis and the isolation of pure compounds for the preparation of analytical standards and for toxicology studies.
To investigate methods for the separation of gram-scale quantities of the epimeric dehydropyrrolizidine alkaloids lycopsamine and intermedine and to compare their NMR spectroscopic data with those of their heliotridine-based analogues echinatine and Rinderine.
METHODS AND RESULTS:
Lycopsamine and intermedine were extracted, predominantly as their N-oxides and along with their acetylated derivatives, from commercial samples of comfrey (Symphytum officinale) root. Alkaloid enrichment involved liquid-liquid partitioning of the crude methanol extract between dilute aqueous acid and n-butanol, reduction of N-oxides and subsequent continuous liquid-liquid extraction of free base alkaloids into CHCl3 . The alkaloid-rich fraction was further subjected to semi-automated flash chromatography using boronated soda glass beads or boronated quartz sand.
Boronated soda glass beads (or quartz sand) chromatography adapted to a Biotage Isolera Flash Chromatography System enabled large-scale separation (at least up to 1-2 g quantities) of lycopsamine and intermedine. The structures were confirmed using one- and two-dimensional (1) H- and (13) C-NMR spectroscopy. Examination of the NMR data for lycopsamine, intermedine and their heliotridine-based analogues echinatine and Rinderine allowed for some amendments of literature data and provided useful comparisons for determining relative configurations in monoester dehydropyrrolizidine alkaloids. A similar NMR comparison of lycopsamine and intermedine with their N-oxides showed the effects of N-oxidation on some key chemical shifts. A levorotatory shift in specific rotation from +3.29° to -1.5° was observed for lycopsamine when dissolved in ethanol or methanol respectively.
CONCLUSIONS:
A semi-automated flash chromatographic process using boronated soda glass beads was standardised and confirmed as a useful, larger scale preparative approach for separating the epimers lycopsamine and intermedine. The useful NMR correlations to stereochemical arrangements within this specific class of dehydropyrrolizidine alkaloid cannot be confidently extrapolated to other similar dehydropyrrolizidine alkaloids.