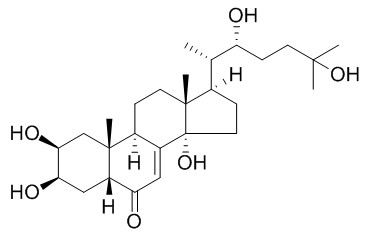
Ecdysone
Ecdysone signaling through Ecdysone receptor isoform B1 is required cell autonomously for the muscle death. A nctional Bombyx Ecdysone receptor binds to EcRE-D and activates the expression of BmBR-C. Mnoaminergic autocrine signaling in the PG regulates Ecdysone biogenesis in a coordinated fashion on activation by PTTH and Ilps.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Funct.2022, 13(14):7638-7649.
Egyptian Pharmaceutical Journal2024, epj_205_23.
VNU Journal of Science2023, No. 20.
Front Immunol. 2020, 11:62.
Molecules.2017, 22(6)
Molecules.2018, 23(3):E615
Front Pharmacol.2018, 9:756
Biomed Pharmacother.2022, 145:112474.
Revista Brasileira de Farmacognosia2024, 34:1156-1165.
J of the Society of Cosmetic Scientists of Korea2018, 44(4):407-417
Related and Featured Products
Dev Biol. 2015 Feb 15;398(2):163-76.
Ecdysone regulates morphogenesis and function of Malpighian tubules in Drosophila melanogaster through EcR-B2 isoform.[Pubmed:
25476260]
Malpighian tubules are the osmoregulatory and detoxifying organs of Drosophila and its proper development is critical for the survival of the organism. They are made up of two major cell types, the ectodermal principal cells and mesodermal stellate cells. The principal and stellate cells are structurally and physiologically distinct from each other, but coordinate together for production of isotonic fluid. Proper integration of these cells during the course of development is an important pre-requisite for the proper functioning of the tubules.
METHODS AND RESULTS:
We have conclusively determined an essential role of Ecdysone hormone in the development and function of Malpighian tubules. Disruption of Ecdysone signaling interferes with the organization of principal and stellate cells resulting in malformed tubules and early larval lethality. Abnormalities include reduction in the number of cells and the clustering of cells rather than their arrangement in characteristic wild type pattern. Organization of F-actin and β-tubulin also show aberrant distribution pattern. Malformed tubules show reduced uric acid deposition and altered expression of Na(+)/K(+)-ATPase pump.
CONCLUSIONS:
B2 isoform of Ecdysone receptor is critical for the development of Malpighian tubules and is expressed from early stages of its development.
Insect Mol Biol. 2014 Jun;23(3):341-56.
Ecdysone response elements in the distal promoter of the Bombyx Broad-Complex gene, BmBR-C.[Pubmed:
24576019]
METHODS AND RESULTS:
As determined by a luciferase assay, the transcriptional activity of Pdist, but not Pprox, was activated by Ecdysone. Further analyses using reporters driven by sequential deletion Pdist mutants indicated that two regions, Ecdysone responsive element (EcRE)-D and EcRE-P, -4950 bp and -3480 bp upstream from the distal transcription start site, respectively, were important in the responsiveness of Pdist to 20-hydroxyEcdysone (20E); however, no significant sequence similarities were found between the canonical EcRE and the EcRE-D or EcRE-P regions. Electrophoretic mobility shift assays showed that both the EcRE-D and -P sequences specifically bound to Bombyx protein(s). Sequence analyses and competition assays suggested that the protein(s) bound to EcRE-P might include components other than the Ecdysone receptor (EcR), suggesting that BmBR-C transcription was indirectly activated by Ecdysone through the EcRE-P. Remarkably, protein binding to the mid-region of the EcRE-D, EcRE-Db, was competitively inhibited by an oligonucleotide containing the Drosophila hsp27 EcRE sequence. Furthermore, an anti-EcR antibody interfered with the formation of the protein-EcRE-Db complex.
CONCLUSIONS:
These results indicated that a functional Bombyx Ecdysone receptor binds to EcRE-D and activates the expression of BmBR-C.
Proc Natl Acad Sci U S A. 2015 Feb 3;112(5):1452-7.
Autocrine regulation of ecdysone synthesis by β3-octopamine receptor in the prothoracic gland is essential for Drosophila metamorphosis.[Pubmed:
25605909]
In Drosophila, pulsed production of the steroid hormone Ecdysone plays a pivotal role in developmental transitions such as metamorphosis. Ecdysone production is regulated in the prothoracic gland (PG) by prothoracicotropic hormone (PTTH) and insulin-like peptides (Ilps).
METHODS AND RESULTS:
Here, we show that monoaminergic autocrine regulation of Ecdysone biosynthesis in the PG is essential for metamorphosis. PG-specific knockdown of a monoamine G protein-coupled receptor, β3-octopamine receptor (Octβ3R), resulted in arrested metamorphosis due to lack of Ecdysone. Knockdown of tyramine biosynthesis genes expressed in the PG caused similar defects in Ecdysone production and metamorphosis. Moreover, PTTH and Ilps signaling were impaired by Octβ3R knockdown in the PG, and activation of these signaling pathways rescued the defect in metamorphosis.
CONCLUSIONS:
Thus, monoaminergic autocrine signaling in the PG regulates Ecdysone biogenesis in a coordinated fashion on activation by PTTH and Ilps. We propose that monoaminergic autocrine signaling acts downstream of a body size checkpoint that allows metamorphosis to occur when nutrients are sufficiently abundant.
Dev Biol. 2013 Nov 15;383(2):275-84.
Ecdysone signaling at metamorphosis triggers apoptosis of Drosophila abdominal muscles.[Pubmed:
24051228]
One of the most dramatic examples of programmed cell death occurs during Drosophila metamorphosis, when most of the larval tissues are destroyed in a process termed histolysis. Much of our understanding of this process comes from analyses of salivary gland and midgut cell death. In contrast, relatively little is known about the degradation of the larval musculature.
METHODS AND RESULTS:
Here, we analyze the programmed destruction of the abdominal dorsal exterior oblique muscle (DEOM) which occurs during the first 24h of metamorphosis. We find that Ecdysone signaling through Ecdysone receptor isoform B1 is required cell autonomously for the muscle death. Furthermore, we show that the orphan nuclear receptor FTZ-F1, opposed by another nuclear receptor, HR39, plays a critical role in the timing of DEOM histolysis. Finally, we show that unlike the histolysis of salivary gland and midgut, abdominal muscle death occurs by apoptosis, and does not require autophagy.
CONCLUSIONS:
Thus, there is no set rule as to the role of autophagy and apoptosis during Drosophila histolysis.
Dev Biol. 2014 Mar 15;387(2):229-39.
INO80-dependent regression of ecdysone-induced transcriptional responses regulates developmental timing in Drosophila.[Pubmed:
24468295]
Sequential pulses of the steroid hormone Ecdysone regulate the major developmental transitions in Drosophila, and the duration of each developmental stage is determined by the length of time between Ecdysone pulses. Ecdysone regulates biological responses by directly initiating target gene transcription. In turn, these transcriptional responses are known to be self-limiting, with mechanisms in place to ensure regression of hormone-dependent transcription. However, the biological significance of these transcriptional repression mechanisms remains unclear.
METHODS AND RESULTS:
Here we show that the chromatin remodeling protein INO80 facilitates transcriptional repression of Ecdysone-regulated genes during prepupal development. In ino80 mutant animals, inefficient repression of transcriptional responses to the late larval Ecdysone pulse delays the onset of the subsequent prepupal Ecdysone pulse, resulting in a significantly longer prepupal stage. Conversely, increased expression of ino80 is sufficient to shorten the prepupal stage by increasing the rate of transcriptional repression. Furthermore, we demonstrate that enhancing the rate of regression of the mid-prepupal competence factor βFTZ-F1 is sufficient to determine the timing of head eversion and thus the duration of prepupal development.
CONCLUSIONS:
Although ino80 is conserved from yeast to humans, this study represents the first characterization of a bona fide ino80 mutation in any metazoan, raising the possibility that the functions of ino80 in transcriptional repression and developmental timing are evolutionarily conserved.