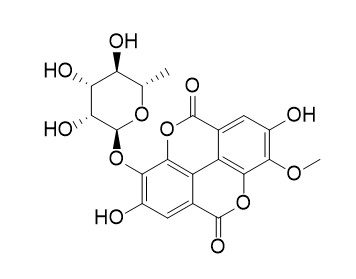
3-O-Methylellagic acid 3'-O-alpha-rhamnopyranoside
3-O-Methylellagic acid 3'-O-α-rhamnopyranoside has antioxidant and antibacterial activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Pharmacol.2019, 10:1355
Curr Issues Mol Biol.2023, 45(2):1587-1600.
Molecules.2018, 23(2)
Sci Rep.2020, 10:4495(2020)
Cell.2018, 172(1-2):249-261
Phytomedicine.2019, 67:153159
Evid Based Complement Alternat Med.2020, 2020:9416962.
LWT2024, 200:116184.
Natural Product Sciences2024, 30(4):309-315.
Acta Chromatographica2021, 00960.
Related and Featured Products
Phytochemistry . 2001 Jun;57(4):587-591.
Ellagic acid rhamnosides from the stem bark of Eucalyptus globulus[Pubmed:
11394863]
Four ellagic acid rhamnosides were isolated from the stem bark of Eucalyptus globulus. Their structures have been established on the basis of the analysis of their 1H NMR, 13C NMR, HMBC, IR and MS spectral data. The HMBC data of these compounds were most useful for their structure determinations, with these bring determined to be 3-O-Methylellagic acid 3'-O-alpha-rhamnopyranoside, 3-O-methylellagic acid 3'-O-alpha-3''-O-acetylrhamnopyranoside, 3-O-methylellagic acid 3'-O-alpha-2''-O-acetylrhamnopyranoside, 3-O-methylellagic acid 3'-O-alpha-4''-O-acetylrhamnopyranoside, respectively. Their antioxidant activities were evaluated by measuring the inhibition of lipid peroxidation using rat liver microsomes, with IC50 values of 10.0-14.0 microg/ml.
Nat Prod Res . 2014;28(4):230-238.
Isolation, characterisation and antibacterial activity of new compounds from methanolic extract of seeds of Caesalpinia crista L. (Caesalpinaceae)[Pubmed:
23822804]
Phytochemical study on the methanolic extract of Caesalpinia crista afforded two novel compounds, 2-hydroxytrideca-3,6-dienyl-pentanoate and octacosa-12,15-diene along with known compounds 3-O-methylellagic acid 3'O-α-rhamnopyranoside, β-sitosterol and sucrose. Compound 3-O-methylellagic acid 3'O-α-rhamnopyranoside is reported for the first time from the plant. Molecular structures, of isolated compounds, were elucidated by using the NMR spectroscopy in combination with IR and mass spectral data. All isolated compounds, extract and fractions were evaluated for in vitro antibacterial activity against various Gram-positive and Gram-negative bacterial strains and found to be significantly active against Staphylococcus aureus and methicillin-resistant S. aureus (minimum inhibitory concentration: 64-512 μg mL(- 1)).
Phytochemistry . 2001 Jun;57(4):587-591.
Ellagic acid rhamnosides from the stem bark of Eucalyptus globulus[Pubmed:
11394863]
Four ellagic acid rhamnosides were isolated from the stem bark of Eucalyptus globulus. Their structures have been established on the basis of the analysis of their 1H NMR, 13C NMR, HMBC, IR and MS spectral data. The HMBC data of these compounds were most useful for their structure determinations, with these bring determined to be 3-O-Methylellagic acid 3'-O-alpha-rhamnopyranoside, 3-O-methylellagic acid 3'-O-alpha-3''-O-acetylrhamnopyranoside, 3-O-methylellagic acid 3'-O-alpha-2''-O-acetylrhamnopyranoside, 3-O-methylellagic acid 3'-O-alpha-4''-O-acetylrhamnopyranoside, respectively. Their antioxidant activities were evaluated by measuring the inhibition of lipid peroxidation using rat liver microsomes, with IC50 values of 10.0-14.0 microg/ml.
Nat Prod Res . 2014;28(4):230-238.
Isolation, characterisation and antibacterial activity of new compounds from methanolic extract of seeds of Caesalpinia crista L. (Caesalpinaceae)[Pubmed:
23822804]
Phytochemical study on the methanolic extract of Caesalpinia crista afforded two novel compounds, 2-hydroxytrideca-3,6-dienyl-pentanoate and octacosa-12,15-diene along with known compounds 3-O-methylellagic acid 3'O-α-rhamnopyranoside, β-sitosterol and sucrose. Compound 3-O-methylellagic acid 3'O-α-rhamnopyranoside is reported for the first time from the plant. Molecular structures, of isolated compounds, were elucidated by using the NMR spectroscopy in combination with IR and mass spectral data. All isolated compounds, extract and fractions were evaluated for in vitro antibacterial activity against various Gram-positive and Gram-negative bacterial strains and found to be significantly active against Staphylococcus aureus and methicillin-resistant S. aureus (minimum inhibitory concentration: 64-512 μg mL(- 1)).