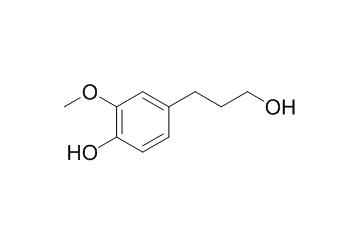
Dihydroconiferyl alcohol
Dihydroconiferyl alcohol exhibits cytoprotective activity in cultured MCF-7 cells stressed by H2O2.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Plants (Basel).2024, 13(19):2793.
Food Chem.2024, 458:140201.
Sci Rep.2021, 11(1):10931.
Mol Microbiol.2019, 112(1):317-332
Journal of Phytopathology2021, 169,Issue11-12.
Curr Issues Mol Biol.2022, 44(10):5106-5116.
Biochem Biophys Res Commun.2018, 505(1):194-200
J Nat Prod.2018, 81(4):966-975
Int J Mol Sci.2022, 23(11):6104.
J Biomol Struct Dyn.2022, 1-21.
Related and Featured Products
J Agric Food Chem. 2008 Sep 10;56(17):7759-64.
Lignans and other constituents of the fruits of Euterpe oleracea (Acai) with antioxidant and cytoprotective activities.[Pubmed:
18656934 ]
METHODS AND RESULTS:
Using a hydroxyl radical scavenging assay, bioactivity-guided fractionation of a methanol-soluble extract of the fruits of Euterpe oleracea (acai) led to the isolation of 22 compounds of previously known structure. Altogether, 14 of these isolates were found to be active in an in vitro hydroxyl radical scavenging assay and seven of these isolates in a 1,1-diphenyl-2-picrylhydrazyl radical scavenging assay. Dihydroconiferyl alcohol, (+)-lariciresinol, (+)-pinoresinol, (+)-syringaresinol, and protocatechuic acid methyl ester exhibited cytoprotective activity in cultured MCF-7 cells stressed by H2O2.
CONCLUSIONS:
Lignans have not been previously reported as constituents of this species and were found to be representative of the aryltetrahydronaphthalene, dihydrobenzofuran, furofuran, 8-O-4'-neolignan, and tetrahydrofuran structural types.
Molecules. 2017 Aug 14;22(8).
Inositol Derivatives and Phenolic Compounds from the Roots of Taraxacum coreanum.[Pubmed:
28805750 ]
METHODS AND RESULTS:
In this study, the characterization of chemical constituents and biological activity of the roots of Taraxacum coreanum (Asteraceae) was attempted. Phytochemical investigation of the roots of T. coreanum led to the isolation of two new inositol derivatives, taraxinositols A (1) and B (2), and a new phenolic compound, taraxinol (16), together with twenty known compounds including four inositol derivatives, neo-inositol-1,4-bis (4-hydroxybenzeneacetate) (3), chiro-inositol-1,5-bis(4- hydroxybenzeneacetate) (4), chiro-inositol-2,3-bis (4-hydroxybenzeneacetate) (5) and chiro-inositol- 1,2,3-tris (4-hydroxybenzeneacetate) (6), nine phenolic compounds: p-hydroxybenzaldehyde (7), vanillin (8), syringaldehyde (9), vanillic acid (10), 4-methoxyphenylacetic acid (11), 4-hydroxy- phenylacetic acid methyl ester (12), optivanin (13), isoferulic acid (14) and Dihydroconiferyl alcohol (15), four coumarins: nodakenetin (17), decursinol (18), prangol (19) and isobyakangelicin (20), and three lignans: syringaresinol-4'-O-β-d-glucoside (21), syringaresinol (22), and pinoresinol (23).
The structures of isolated compounds were determined on the basis of spectroscopic analysis.
CONCLUSIONS:
Among the isolated compounds, vanillic acid, isoferulic acid and syringaresinol showed radical scavenging activity with IC50 values ranging from 30.4 to 75.2 μM.