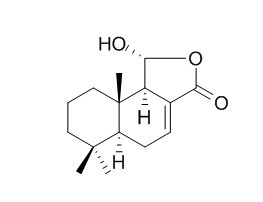
Dendocarbin A
Standard reference
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Antioxidants (Basel).2023, 12(1):189.
Br J Pharmacol.2018, 175(6):902-923
Int J Mol Sci.2024, 25(18):9909.
Molecules.2024, 29(16):3976.
J Food Sci.2022, 87(11):4905-4916.
Plants (Basel).2024, 13(6):868.
Molecules.2019, 24(17):E3127
Biomed Pharmacother.2023, 162:114617.
J of Apicultural Research2020, 10.1080
Food Research International2023, 113792.
Related and Featured Products
Molecules. 2009 Sep 28;14(10):3844-50.
Ugandenial A, a new drimane-type sesquiterpenoid from Warburgia ugandensis.[Pubmed:
19924033]
METHODS AND RESULTS:
One new drimane-type sesquiterpenoid, named ugandenial A (1), was isolated from the ethyl acetate extract of the bark of Warburgia ugandensis (Canellaceae) together with eight known drimane-type sesquiterpenoids: 11alpha-hydroxycinnamosmolide (2), warburganal (3), polygodial (4), mukaadial (5), Dendocarbin A (6), 9alpha-hydroxycinnamolide (7), dendocarbin L (8) and dendocarbin M (9). Their structures were established by detailed spectroscopic analysis. In addition a keto-enol equilibrium was demonstrated for compound 1 through a detailed NMR analysis run in CD(2)Cl(2) at 190 K. Cytotoxicity of the isolated compounds against KB cells was evaluated.
Acta Crystallogr C Struct Chem. 2015 Apr;71(Pt 4):294-7.
A monoclinic form of dendocarbin A: a borderline case of one-dimensional isostructural polymorphism.[Pubmed:
25836288]
The title compound, Dendocarbin A [systematic name: (1R,5aS,9aS,9bR)-1-hydroxy-6,6,9a-trimethyldodecahydronaphtho[1,2-c]furan-3-one], C15H22O3, is a sesquiterpene lactone isolated from Drimys winteri var chilensis.
METHODS AND RESULTS:
The monoclinic phase described herein displays an identical molecular structure to the orthorhombic phase that we reported previously [Paz Robles et al. (2014). Acta Cryst. C70, 1007-1010], while varying significantly in chain pitch, and can thus be considered as a borderline case of one-dimensional isostructural polymorphism.
Acta Crystallogr C Struct Chem. 2014 Nov;70(Pt 11):1007-10.
Dendocarbin A: a sesquiterpene lactone from Drimys winteri.[Pubmed:
25370095]
The natural compound Dendocarbin A, C15H22O3, is a sesquiterpene lactone isolated for the first time from Drimys winteri for var chilensis.
METHODS AND RESULTS:
The compound crystallizes in the orthorhombic space group P2₁2₁2₁ and its X-ray crystal structure confirmed the S/R character of the chiral centres at C-5/C-10 and C-9/C-11, respectively. The α-OH group at C-11 was found to be involved in intermolecular hydrogen bonding, defining chains along the <100> 2₁ screw axis.