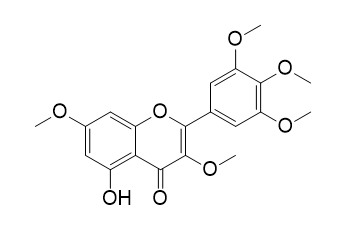
Combretol
Combretol has leishmanicidal activity, it showed moderate activity (growth inhibition 87.3 and 73.0 %, respectively, at 50 µM) against a multidrug-resistant L. tropica line. Combretol also shows weakly active cytotoxic activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biomed Pharmacother.2024, 179:117365.
Antioxidants (Basel).2020, 9(2): E119
Industrial Crops and Products2022, 188:115638
Molecules.2024, 29(5):1050.
Oncol Rep.2019, 41(4):2453-2463
Phytother Res.2022, 10.1002:ptr.7592.
Bioorg Med Chem.2018, 26(14):4201-4208
Fitoterapia.2018, 124:92-102
Phytomedicine Plus2022, 2(1):100207.
Nat Prod Commun.2017, 12(5):771-778
Related and Featured Products
Planta Med. 2011 Jan;77(1):77-80.
Leishmanicidal and reversal multidrug resistance constituents from Aeonium lindleyi.[Pubmed:
20665372 ]
METHODS AND RESULTS:
A new bicyclic diterpene with a labdane skeleton, 7-oxo-labd-8-en-15-ol ( 1), along with two known diterpenes and ten flavonoids were isolated from the leaves of Aeonium lindleyi (Crassulaceae). Their structures were elucidated on the basis of spectroscopic data, including 1D and 2D NMR experiments, and comparison with spectroscopic data reported in the literature.
CONCLUSIONS:
Labdan-8 α,15-diol (2) and labd-8(17)-en-3 β,15-diol (3) showed leishmanicidal activity against Leishmania tropica (IC (50) = 77.0 µM) and Leishmania braziliensis (IC (50) = 68.0 µM) similar to ketoconazole used as positive control. 5,3'-Dihydroxy-3,7,4',5'-tetramethoxyflavone (8) and Combretol (9) showed moderate activity (growth inhibition 87.3 and 73.0 %, respectively, at 50 µM) against a multidrug-resistant L. tropica line.
J Nat Prod. 2006 Feb;69(2):287-9.
Cytotoxic diterpenes from Cassipourea madagascariensis from the Madagascar rainforest.[Pubmed:
16499334 ]
METHODS AND RESULTS:
Bioassay-directed fractionation of ethanol extracts of the roots and leaves of the plant Cassipourea madagascariensis resulted in the isolation of the two new terpenoids cassipourol (1) and cassipouryl acetate (2) in addition to the three known compounds, 3beta,30-dihydroxylup-20(29)-ene (3), 30-hydroxylup-20(29)-en-3-one (4), and Combretol (5). The structures of the two new compounds were established on the basis of 1D and 2D NMR spectroscopic data and chemical conversion.
CONCLUSIONS:
All the isolated compounds were tested against the A2780 human ovarian cancer cell line; the two diterpenes (1 and 2) showed moderate cytotoxic activity, while the three known compounds (3-5) were weakly active.