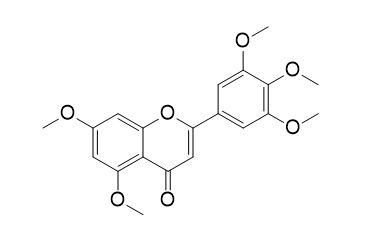
5,7,3',4',5'-Pentamethoxyflavone
3',4',5',5,7-Pentamethoxyflavone has anti-inflammatory and cancer chemopreventive activities. 3',4',5',5,7-Pentamethoxyflavone could be used as an effective adjuvant sensitizer to increase the efficacy of chemotherapeutic drugs by downregulating Nrf2 signaling pathway, it
sensitizes Cisplatin-resistant A549 cells to Cisplatin by inhibition of Nrf2 pathway.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Bone Miner Res.2017, 32(12):2415-2430
Chinese J of Tissue Engineering Res.2022, 26(17): 2636-2641.
Molecules2022, 27(3),1140.
Nutrients.2019, 12(1)
J Ethnopharmacol.2017, 209:305-316
Int J Mol Sci.2019, 20(3):E651
Natural Product Sciences2023, 29(4):276-280.
Academic J of Second Military Medical University2019, 40(1)
Biomed Chromatogr.2016, 30(10):1573-81
Dental Journal2024, 57(4): 254-258
Related and Featured Products
Evid Based Complement Alternat Med. 2018 Dec 2;2018:9806160.
Apoptosis Effects of Dihydrokaempferol Isolated from Bauhinia championii on Synoviocytes.[Pubmed:
30622621 ]
Bauhinia championii (Benth.) Benth. is a traditional medicinal plant used in China to treat rheumatoid arthritis (RA), especially in She ethnic minority group. This study focused on the active constituents from the rattan of B. championii (Benth.) Benth., which possess potential apoptosis effects.
METHODS AND RESULTS:
A conventional phytochemical separation method for the isolation of compounds from the ethyl acetate extract of B. championii was developed. The procedure involved extraction, liquid-liquid partitioning with ethyl acetate, and subsequent compound purification, respectively. Additionally, cell viability of dihydrokaempferol found abundantly in it was evaluated in vitro by MTS, and the antiapoptosis effect was evaluated by annexin V/PI staining (Flow Cytometry Analysis) and western blot. The results showed that nine flavonoids, and five other compounds, were isolated from the ethyl acetate extract of B. championii and were identified as β-sitosterol (1), 5,6,7,3',4',5'-hexamethoxyflavone (2), 3',4',5,7-tetrahydroxyflavone (3), 5,7,3',4',5'-Pentamethoxyflavone (4), 4'-hydroxy-5,7,3',5'-pentamethoxyflavone (5), apigenin (6), liquiritigenin (7), 5, 7-dihydroxylcoumarin (8), 3',4',5,7, -pentamethoxyflavone (9), n-octadecanoate (10), lupine ketone (11), dibutylphthalate (12), dihydrokaempferol (13), and 5,7,3',5'-tetrahydroxy-6-methylflavanone (14). Among these compounds, 5-14 were isolated for the first time from B. championii. In addition, apoptosis effects of abundant dihydrokaempferol were evaluated in vitro. Dihydrokaempferol exhibited inhibitory effects on the proliferation of synoviocytes. Furthermore, dihydrokaempferol promoted Bax and Bad expression, as well as the cleavage of caspase-9, caspase-3, and PARP. Meanwhile, it inhibited Bcl-2 and Bcl-xL expression.
CONCLUSIONS:
These findings indicate that dihydrokaempferol isolated from the ethyl acetate extract of B. championii effectively promotes apoptosis, which is an important process through suppression of apoptotic activity. The results are encouraging for further studies on the use of B. championii in the treatment of RA.
Cancer Prev Res (Phila). 2009 Aug;2(8):743-50.
Flavones as colorectal cancer chemopreventive agents--phenol-o-methylation enhances efficacy.[Pubmed:
19638489 ]
Flavonoids occur ubiquitously in plants, and some possess preclinical cancer chemopreventive activity. Little is known about molecular features that mediate chemopreventive efficacy of flavonoids.
METHODS AND RESULTS:
Here, three related flavones, apigenin (4',5,7-trihydroxyflavone), tricin (4',5,7-trihydroxy-3',5'-dimethoxyflavone), and 3',4',5',5,7-pentamethoxyflavone (5,7,3',4',5'-Pentamethoxyflavone,PMF), were compared in terms of their effects on (a) adenoma development in Apc(Min) mice, a model of human gastrointestinal malignancies; (b) growth of APC10.1 mouse adenoma cells in vitro; and (c) prostaglandin E-2 generation in HCA-7 human-derived colorectal cancer cells in vitro. Life-long consumption of PMF with the diet at 0.2% reduced Apc(Min) mouse adenoma number and burden by 43% and 61%, respectively, whereas apigenin was inactive. Tricin has previously shown activity in this model. IC50 values for murine adenoma cell growth inhibition by PMF, tricin, and apigenin were 6, 13, and 18 micromol/L, respectively. In Apc(Min) mice that received flavones (0.2%) for 4 weeks, adenoma cell proliferation as reflected by Ki-67 staining was reduced by PMF and tricin, but not by apigenin. On incubation with HCA-7 cells for 6 hours, PMF reduced prostaglandin E-2 generation with an IC50 of 0.8 micromol/L, a fraction of the respective values reported for tricin or apigenin. In silico PMF docked into the cyclooxygenase active site with greater affinity than tricin or apigenin.
CONCLUSIONS:
The results suggest that the rank order of cancer chemopreventive efficacy in Apc(Min) mice is PMF > tricin > apigenin, supporting the notion that the presence of O-methyl in the flavone molecular scaffold promotes gastrointestinal cancer chemopreventive efficacy.
Mol Cells. 2015 May;38(5):396-401.
3',4',5',5,7-pentamethoxyflavone sensitizes Cisplatin-resistant A549 cells to Cisplatin by inhibition of Nrf2 pathway.[Pubmed:
25843086 ]
Nuclear factor erythroid 2-related factor 2 (Nrf2) is an important redox-sensitive transcription factor that regulates the expression of several cytoprotective genes. More recently, genetic analyses of human tumors have indicated that Nrf2 may cause resistance to chemotherapy.
METHODS AND RESULTS:
In this study, we found that the expression levels of Nrf2 and its target genes GCLC, HO-1, NQO1 were significantly higher in cisplatin-resistant A549 (A549/CDDP) cells than those in A549 cells, and this resistance was partially reversed by Nrf2 siRNA. 3',4',5',5,7-Pentamethoxyflavone (5,7,3',4',5'-Pentamethoxyflavone,PMF), a natural flavonoid extracted from Rutaceae plants, sensitized A549/CDDP to CDDP and substantially induced apoptosis compared with that of CDDP alone treated group, and this reversal effect decreased when Nrf2 was downregulated by siRNA. Mechanistically, PMF reduced Nrf2 expression leading to a reduction of Nrf2 downstream genes, and in contrast, this effect was decreased by blocking Nrf2 with siRNA.
CONCLUSIONS:
Taken together, these results demonstrated that PMF could be used as an effective adjuvant sensitizer to increase the efficacy of chemotherapeutic drugs by downregulating Nrf2 signaling pathway.
Pharm Biol. 2016;54(5):868-81.
The anti-inflammatory activity of several flavonoids isolated from Murraya paniculata on murine macrophage cell line and gastric epithelial cell (GES-1).[Pubmed:
26710980 ]
Context Murraya paniculata (L.) Jack (Rutaceae), Qianlixiang in Chinese, is distributed in China. As an important traditional Chinese medicine (TCM), it demonstrates many bioactivities, such as febrifuge, astringent, anti-dysenteric, and tonic.
The objective of this study is to evaluate the anti-inflammatory effect of three flavonoids isolated from M. paniculata in lipopolysaccharide (LPS)-activated murine macrophage cell line and ethanol-induced gastric damage on gastric epithelial cell (GES-1).
METHODS AND RESULTS:
Three identified flavonoids were isolated from stems and leaves of M. paniculata using ultra performance liquid chromatography (UPLC). Cell viability was measured with MTT, mouse peritoneal macrophages and GES-1 cells were incubated with 0, 0.01, 0.1, 1, 10, and 100 μM P1, P3 and P8 for 24, 48, and 72 h. The inhibitory effect of pretreatment with various concentrations of 5,7,3',4',5'-Pentamethoxyflavone (P1), 5,7,3',4'-tetramethoxyflavone (P3), or 5-desmethylnobiletin 5-hydroxy-6,7,8,3',4'-pentameth-oxyflavone (P8) ranging from 0.03 to 30 μM on nitric oxide (NO) secretion was quantified by the Griess assay for 24 and 48 h, while interleukin-6 (IL-6) was measured by ELISA for 24 and 48 h. The effects of P1, P3, and P8 on mouse peritoneal macrophages and GES-1 cells were not attributable to cytotoxic effects at the doses of 0-10 μM. The IC50 value of P1 is 53.40 μM, P3 is 120.98 μM, and P8 is 10.73 μM. The concentration of the three flavonoids had the best effects of anti-inflammation upon NO inhibition at the dose of 3 μM. P3 had the highest inhibition on IL-6 production. The GES-1 cells pretreated with three flavonoids showed a significant increase in the level of NO (P1: 7.94 ± 0.0635 μM, P3: 8.81 ± 0.0159 μM, and P8: 8.51 ± 0.0522 μM) at 24 h and a more significant increase at 48 h (P1: 9.34 ± 0.0975 μM, P3: 11.9 ± 0.0672 μM, and P8: 9.34 ± 0.0454 μM).
CONCLUSIONS:
The current results suggested that the anti-inflammatory activity of three flavonoids was mainly manifested in the reduction of production of NO and IL-6 production. Analysis of the structure-activity relationship indicated that the double bond at C2-C3 and the position of the B ring at C2/C3 seemed to be indispensable for the anti-inflammatory activity.
Biochem Biophys Res Commun. 2004 Jul 30;320(3):672-9.
Reversal of P-glycoprotein-mediated MDR by 5,7,3',4',5'-pentamethoxyflavone and SAR.[Pubmed:
15240100 ]
METHODS AND RESULTS:
During screening for the flavonoid chemosensitizers, it was found that 5,7,3',4',5'-Pentamethoxyflavone (PMF) was equipotent to verapamil in vitro with respect to the chemosensitizing effect. PMF appears to have a chemosensitizing effect not only by increasing the intracellular accumulation of the drugs without competition in a binding site of azidopine but also by interfering with the substrate-stimulated ATPase activity. Structure-activity relationship suggests that methoxylated substitution and its numbers or sites of the rings are more important than its hydroxylated counterparts in chemosensitization.
CONCLUSIONS:
Overall, PMF is anticipated to be a novel and highly potent second-generation flavonoid chemosensitizer because PMF has significant advantages of having a high therapeutic index, of being a non-transportable inhibitor, and of having a low possibility of drug interactions at the azidopine-binding site of Pgp.