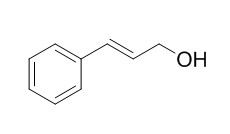
Cinnamyl alcohol
Cinnamyl alcohol could as a fragrance ingredient with no safety concerns, it readily autoxidizes upon air exposure, and forms strong sensitizers as determined by the local lymph node assay.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Mol Pharm.2018, 15(8):3285-3296
Int J Mol Sci.2022, 23(13):7115.
Nat Commun.2019, 10(1):2745
Sci Rep.2023, 13(1):13610.
Journal of Food Quality2022, P:13, 6256310.
Phytomedicine.2022, 96:153877.
Nat Prod Sci.2014, 20(3):182-190
J Mol Histol.2019, 50(4):343-354
Int J Mol Sci.2022, 23(23):15213.
Toxins (Basel).2021, 13(9):593.
Related and Featured Products
Contact Dermatitis. 2013 Mar;68(3):129-38.
Cinnamyl alcohol oxidizes rapidly upon air exposure.[Pubmed:
23421457]
Cinnamyl alcohol and cinnamal are frequent fragrance contact allergens. Both are included in the European baseline fragrance mix I, which is used for screening of contact allergy in dermatitis patients. The aim of this study was to investigate the autoxidation of Cinnamyl alcohol and to identify the oxidation products formed on air exposure. We also wanted to evaluate the effect of autoxidation on the sensitization potency of Cinnamyl alcohol.
METHODS AND RESULTS:
Samples of commercially available Cinnamyl alcohol with and without purification were exposed to air, and the autoxidation was followed by chemical analysis. The analysis was performed with mass spectrometry (LC/MS/MS). Sensitization potencies of compounds were determined with the murine local lymph node assay (LLNA) in mice. Chemical analysis showed that the concentration of Cinnamyl alcohol in the air-exposed samples decreased rapidly over time, and that autoxidation products were formed. Cinnamal, epoxy Cinnamyl alcohol and cinnamic acid were identified as oxidation products. According to our study, cinnamal and epoxy Cinnamyl alcohol were the first autoxidation products formed. The epoxy Cinnamyl alcohol was shown to be the oxidation product with the highest sensitization potency. The analysis of our samples of commercially available Cinnamyl alcohol showed that there was already a content of 1.5% cinnamal at the start of the autoxidation experiments.
CONCLUSIONS:
Cinnamyl alcohol readily autoxidizes upon air exposure, and forms strong sensitizers as determined by the LLNA. Cinnamal was formed in the largest amounts, showing that cinnamal is not only formed via bioactivation, as has previously been shown. A highly sensitizing epoxide was also identified and quantified in the oxidation mixture.
Food Chem Toxicol. 2007;45 Suppl 1:S1-23.
A toxicologic and dermatologic assessment of related esters and alcohols of cinnamic acid and cinnamyl alcohol when used as fragrance ingredients.[Pubmed:
18035463 ]
A toxicologic and dermatologic assessment of related esters and alcohols of cinnamic acid and Cinnamyl alcohol when used as fragrance ingredients.
Chemistry. 2014 Apr 25;20(18):5478-86.
Highly efficient direct aerobic oxidative esterification of cinnamyl alcohol with alkyl alcohols catalysed by gold nanoparticles incarcerated in a nanoporous polymer matrix: a tool for investigating the role of the polymer host.[Pubmed:
24644103]
METHODS AND RESULTS:
The selective aerobic oxidation of Cinnamyl alcohol to cinnamaldehyde, as well as direct oxidative esterification of this alcohol with primary and secondary aliphatic alcohols, were achieved with high chemoselectivity by using gold nanoparticles supported in a nanoporous semicrystalline multi-block copolymer matrix, which consisted of syndiotactic polystyrene-co-cis-1,4-polybutadiene. The cascade reaction that leads to the alkyl cinnamates occurs through two oxidation steps: the selective oxidation of Cinnamyl alcohol to cinnamaldehyde, followed by oxidation of the hemiacetal that results from the base-catalysed reaction of cinnamaldehyde with an aliphatic alcohol. The rate constants for the two steps were evaluated in the temperature range 10-45 °C. The Cinnamyl alcohol oxidation is faster than the oxidative esterification of cinnamaldehyde with methanol, ethanol, 2-propanol, 1-butanol, 1-hexanol or 1-octanol. The rate constants of the latter reaction are pseudo-zero order with respect to the aliphatic alcohol and decrease as the bulkiness of the alcohol is increased. The activation energy (Ea) for the two oxidation steps was calculated for esterification of Cinnamyl alcohol with 1-butanol (Ea = 57.8±11.5 and 62.7±16.7 kJ mol(-1) for the first and second step, respectively). The oxidative esterification of Cinnamyl alcohol with 2-phenylethanol follows pseudo-first-order kinetics with respect to 2-phenylethanol and is faster than observed for other alcohols because of fast diffusion of the aromatic alcohol in the crystalline phase of the support.
CONCLUSIONS:
The kinetic investigation allowed us to assess the role of the polymer support in the determination of both high activity and selectivity in the title reaction.