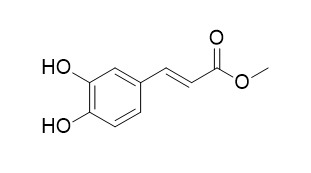
Methyl caffeate acid
Methyl caffeate acid shows anti-complementary activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Plant Commun.2024, 5(10):101005.
Foods2023, 12(23), 4342.
Revista Brasileira de Farmacognosia2024, 34:1091-1100.
J Health Sci Med Res.2023, 31584.
Evid Based Complement Alternat Med.2018, 2018:4580627
Acta Physiologiae Plantarum2016, 38:7
Comput Biol Med.2024, 178:108775.
Srinakharinwirot University2023, 2669.
Green Chem.2023, 25:5222-5232
Applied Biological Chemistry2024, 67:66.
Related and Featured Products
Zhongguo Zhong Yao Za Zhi. 2015 Jan;40(2):269-74.
Anti-complementary phenolic acids from Lonicera japonica.[Pubmed:
26080557]
To study the anti-complementary phenolic acids from Lonicera japonica.
METHODS AND RESULTS:
The anti-complementary activity-directed isolation was carried out with the hemolysis test as guide. All isolation was evaluated for their in vitro anti-complementary activities. The structures were identified by various spectroscopic data including ESI-MS, 1H-NMR, 13C-NMR data.
Fourteen compounds were isolated from the EtOAc fraction of L. japonica extracts, including 8 phenolic acids: 5-O-caffeoylquinic acid (1), chlorogenic (2), 4-O-caffeoylquinic acid (3), 3,5-di-O-caffeoylquinic acid (4), 4,5-di-O-caffeoylquinic acid (5), 3,4-di-O-caffeoylquinic acid (6), caffeic acid (7) and Methyl caffeate acid (8); 3 iridoids: secologanoside (9), sweroside (10) and secoxyloganin (11); and 3 flavonoids: luteolin (12), quercetin (13) and kaempferol (14). Compounds 1-9 and 11-14 showed anti-complementary activity in different extents and 3,5-di-O-caffeoylquinic acid (4) exhibited the most significant activity against the classical pathway.
CONCLUSIONS:
Compound 14 is obtained from this plant for the first time, phenolic acids are the main anti-complementary constituents of L. japonica and 3,5-di-O-caffeoylquinic acid(4) is a potential complement inhibitor with strong activity, which worthy to be studied further in the future.
Chinese Traditional and Herbal Drugs, 2016.
Chemical constituents from aerial parts of Fagopyrum dibotrys.[Reference:
WebLink]
To study the chemical constituents from the aerial parts of Fagopyrum dibotrys.
METHODS AND RESULTS:
The compounds were isolated and purified by means of chromatographic techniques and their structures were identified on the basis of spectral features. Fourteen known compounds were isolated in the methanol extract from the aerial parts of F. dibotrys and their structures were identified as benzoic acid (1), p-hydroxybenzoic acid (2), p-hydroxy benzaldehyde (3), 3,4-dihydroxy benzoic acid (4), succinic acid (5), caffeic acid (6), Methyl caffeate acid (7), luteolin (8), tricin (9), quercetin (10), afzelin A (11), 2α,3β,29-trihydroxyolean-12-en-28-oic acid (12), yarumic acid (13), and 3α-hydroxy-urs-12,15-dien (14).
CONCLUSIONS:
Compounds 2-3 and 6-9 are firstly obtained from the aerial parts of F. dibotrys. Compounds 11-14 are isolated from the genus of Fagopyrum Mill. for the first time.