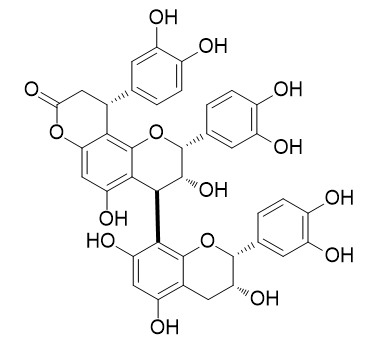
Cinchonain IIa
Cinchonain IIa has antioxidant activity, it shows high radical scavenging activity and reducing power.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytomedicine.2018, 47:48-57
Eur J Pharmacol.2024, 978:176800.
Tumour Biol.2015, 36(12):9385-93
Food Res Int.2020, 133:109130.
Life Sci.2021, 270:119074.
Int J Mol Sci.2018, 19(9):E2528
Regen Biomater.2023, 10:rbad077.
J Mol Histol.2019, 50(4):343-354
Phytomedicine.2018, 40:37-47
Korean Journal of Pharmacognosy2018, 49(4):349-361
Related and Featured Products
Chemical & Pharmaceutical Bulletin, 2008, 33(8):3142-3152.
Tannins and related compounds. XXXI. Isolation and characterization of proanthocyanidins in Kandelia candel (L.) Druce.[Reference:
WebLink]
METHODS AND RESULTS:
One of the species of commercially available catuaba was identified as Anemopaegma arvense by comparison of its micromorphological characteristics and TLC profile with six species of authenticated plants that are commonly referred to as catuaba. The bioactivity-guided fractionation of the ethyl acetate extract of the stem bark of this catuaba sample resulted in the isolation of one new (1, catuabin A) and three known flavan-3-ol type phenylpropanoids, cinchonain Ia (2), Cinchonain IIa (3), and kandelin A1 (4) with antioxidant activities.
CONCLUSIONS:
The structures of these compounds were determined by a combination of spectroscopic techniques. Additionally, these compounds were tested for their anti-inflammatory, cytotoxicity, antimalarial, and antimicrobial activities, where no activity was observed.
Journal of the Brazilian Chemical Society, 2011, 22(11):2087-2093.
Phenylpropanoid Substituted Flavan-3-ols from Trichilia catigua and their In Vitro Antioxidative Activity[Reference:
WebLink]
METHODS AND RESULTS:
The new phenylpropanoid substituted flavan-3-ol apocynin E, together with eight known compounds, epicatechin, procyanidin B2, procyanidin B4, procyanidin C1, cinchonain Ia, cinchonain Ib, cinchonain IIb, and Cinchonain IIa were isolated from an acetone-H2O extract of the air-dried stem bark of Trichilia catigua. The cinchonain Ia e Ib were reevaluated to its estereochemistry. All the compounds were characterized by spectroscopic analysis including 1D and 2D nuclear magnetic resonance (NMR) and mass spectrometry (MS) of their peracetate derivatives. The absolute configuration of the phenylpropanoid moiety was determined by circular dichroism (CD) spectra and by analyzing the anisotropic effects in the Dreiding model and nuclear Overhauser effect (NOESY NMR) experiments.
CONCLUSIONS:
The nine isolated compounds showed higher radical scavenging activity and reducing power than ascorbic acid and Trolox in the free-radical 2,2-diphenyl-1-picrylhydrazyl and Fe3+-Fe2+ reduction assay systems.
Planta Med. 2007 Aug;73(10):1107-11.
Flavan-3-ol-phenylpropanoid conjugates from Anemopaegma arvense and their antioxidant activities.[Pubmed:
17642036 ]
METHODS AND RESULTS:
One of the species of commercially available catuaba was identified as Anemopaegma arvense by comparison of its micromorphological characteristics and TLC profile with six species of authenticated plants that are commonly referred to as catuaba. The bioactivity-guided fractionation of the ethyl acetate extract of the stem bark of this catuaba sample resulted in the isolation of one new (1, catuabin A) and three known flavan-3-ol type phenylpropanoids, cinchonain Ia (2), Cinchonain IIa (3), and kandelin A1 (4) with antioxidant activities.
CONCLUSIONS:
The structures of these compounds were determined by a combination of spectroscopic techniques. Additionally, these compounds were tested for their anti-inflammatory, cytotoxicity, antimalarial, and antimicrobial activities, where no activity was observed.