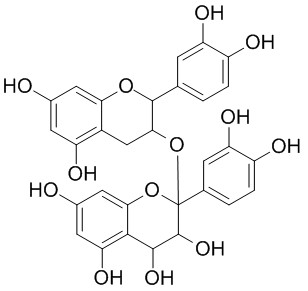
Proanthocyanidins
Proanthocyanidins exerts antiviral, hepatoprotective, cardioprotective, hypolipidemic, anti-osteoporosis, anti-inflammatory ,antioxidant and anti-apoptosis activities. Dietary proanthocyanidins inhibits photocarcinogenesis in mice through the inhibition of UVB-induced inflammation and mediators of inflammation in mouse skin.
Proanthocyanidins form stable complexes with metal ions and with proteins and are good reducing agents, they may participate in the prevention of cancers, both of the digestive tract and inner organs, they may also protect LDLs against oxidation and inhibit platelet aggregation and therefore prevent cardiovascular diseases.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Chemistry of Plant Materials.2019, 215-222
Am J Chin Med.2023, 51(7):1675-1709.
Rev. Chim.2020, 71(3),558-564
Molecules.2022, 27(7):2360.
Evid Based Complement Alternat Med.2017, 2017:6360836
Chemical Engineering Journal2024, 500:157110
Plant Biotechnol (Tokyo).2024, 41(3):267-276.
Protoplasma.2024, 261(6):1267-1280.
Chinese Pharmacological Bulletin2019, 35(8):1120-1125
Evid Based Complement Alternat Med.2021, 2021:8707280.
Related and Featured Products
J Toxicol Sci. 2014;39(5):803-13.
Grape seed proanthocyanidins inhibit H2O2-induced osteoblastic MC3T3-E1 cell apoptosis via ameliorating H2O2-induced mitochondrial dysfunction.[Pubmed:
25242411]
Oxidative stress represents a major cause of cellular damage and death in pathological conditions including osteoporosis, in which oxidative stress is associated with increased bone resorption and low bone mass. And grape seed Proanthocyanidins are a group of polyphenolic bioflavonoids which are known to possess broad pharmacological activity and therapeutic potential, exerting a protective role against oxidant injury. The aim of our study was to investigate whether Proanthocyanidins exert an anti-apoptosis effect in osteoblastic MC3T3-E1 cells, via their antioxidant activity.
METHODS AND RESULTS:
Firstly, we determined the anti-apoptosis effect of Proanthocyanidins in osteoblastic MC3T3-E1 cells, which were subject to H2O2 treatment, then we determined the association of the antioxidant activity exerted by Proanthocyanidins with their anti-apoptosis effect. Results demonstrated that Proanthocyanidins inhibit H2O2-promoted apoptosis in MC3T3-E1 cells, via ameliorating the viability of MC3T3-E1 cells post H2O2 treatment and reducing the apoptotic cell numbers. And the Proanthocyanidins treatment also ameliorates the H2O2-induced mitochondrial dysfunction via promoting the mitochondrial membrane potential (MMP) and respiratory chain complex IV, and reducing the mitochondrial free radical production, ROS and mitochondrial superoxide. Moreover, the Proanthocyanidins inhibit H2O2-induced apoptosis signaling which is mediated by p53.
CONCLUSIONS:
This study implied a possible anti-osteoporosis effect of Proanthocyanidins via their antioxidant and anti-apoptosis activity.
J. Sci. Food Agric., 2000, 80(7):1094-17.
Proanthocyanidins and tannin-like compounds--nature, occurrence, dietary intake and effects on nutrition and health.[Reference:
WebLink]
Proanthocyanidins (syn condensed tannins) are complex flavonoid polymers naturally present in cereals, legume seeds and particularly abundant in some fruits and fruit juices. They share some common structural features--phenolic nature and high molecular weight--with phenolic polymers found in black tea and red wine (called here tannin-like compounds). The polymeric nature of Proanthocyanidins makes their analysis and estimation in food difficult. For this reason, little is known about their consumption, although they likely contribute a large part of the daily polyphenol intake. They also share common physicochemical properties: they form stable complexes with metal ions and with proteins and are, like other polyphenols, good reducing agents. Many of their biological effects of nutritional interest derive from these properties. As metal ion chelators, they influence the bioavailability of several minerals. The nutritional significance of the non-specific complexation of proteins is less clear. As reducing agents, they may participate in the prevention of cancers, both of the digestive tract and inner organs. They may also protect LDLs against oxidation and inhibit platelet aggregation and therefore prevent cardiovascular diseases.
CONCLUSIONS:
In vitro, animal and human studies on the prevention of these chronic diseases are reviewed with particular attention to wine and tea polyphenols. The lack of data on their bioavailability and the paucity of human studies are emphasised.
Planta Med. 2002 Jun;68(6):539-41.
Antiviral and antioxidant activity of flavonoids and proanthocyanidins from Crataegus sinaica.[Pubmed:
12094299 ]
The antiviral and antioxidant activity of some fractions and of a series of flavonoids and Proanthocyanidins obtained from Crataegus sinaica (Rosaceae) was evaluated.
METHODS AND RESULTS:
The O-glycosidic flavonoids and the oligomeric Proanthocyanidins exhibited significant inhibitory activity against herpes simplex virus type 1 (HSV-1), which was shown to be due to an extracellular mechanism for procyanidin C-1. Procyanidin C-1 also had the highest antioxidant activity in both the microsomal lipid peroxidation and the hydroxyl radical scavenging assay. In addition to the previously reported phenolic compounds, the pentacyclic triterpenoid ursolic acid (1) and a tetrameric (2) and pentameric procyanidin (3) are reported for the first time.
J Med Food. 2014 Jun;17(6):663-9.
Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (CCl4)-induced steatosis and liver injury in rats via CYP2E1 regulation.[Pubmed:
24712752]
Liver steatosis is characterized by lipid dysregulation and fat accumulation in the liver and can lead to oxidative stress in liver. Since Proanthocyanidins are present in plant-based foods and have powerful antioxidant properties, we investigated whether Proanthocyanidins can prevent oxidative stress and subsequent liver injury. Carbon tetrachloride (CCl4) treatment can cause steatosis in rats that models both alcoholic and non-alcoholic fatty liver disease in humans.
METHODS AND RESULTS:
We pre-treated rats by oral administration of Proanthocyanidins extracted from grape seeds 7 days prior to intragastrically administering CCl4. Proanthocyanidin treatment continued for an additional 2 weeks, after which time liver and serum were harvested, and mediators of liver injury, oxidative stress, and histological features were evaluated. CCl4-treated rats exhibited significant increases in the following parameters as compared to non-treated rats: fat droplets in the liver, liver injury (ALT, AST), and DNA damage (8-OHdG). Additionally, CCl4 treatment decreased antioxidant enzymes SOD, GSH, GPX, and CAT in the liver due to their rapid depletion after battling against oxidative stress. Compared to CCl4-treated rats, treatment with Proanthocyanidins effectively suppressed lipid accumulation, liver injury, DNA damage, as well as restored antioxidant enzyme levels. Further investigation revealed that Proanthocyanidins treatment also inhibited expression of CYP2E1 in liver, which prevented the initial step of generating free radicals from CCl4.
CONCLUSIONS:
The data presented here show that treatment with orally administered Proanthocyanidins prevented liver injury in the CCl4-induced steatosis model, likely through exerting antioxidant actions to suppress oxidative stress and inhibiting the free radical-generating CYP2E1 enzyme.
Bladé C, Arola L, Salvadó M J. Mol. Nutr. Food Res.,2010 Jan;54(1):37-59.
Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms[Pubmed:
19960459]
Proanthocyanidins are the most abundant polyphenols in human diets. Epidemiological studies strongly suggest that Proanthocyanidins protect against cardiovascular diseases. Despite the antioxidant and anti-inflammatory properties of these flavonoids, one of the mechanisms by which Proanthocyanidins exert their cardiovascular protection is improving lipid homeostasis. Animal studies demonstrate that Proanthocyanidins reduce the plasma levels of atherogenic apolipoprotein B-triglyceride-rich lipoproteins and LDL-cholesterol but increase antiatherogenic HDL-cholesterol. The results in humans, however, are less clear.
METHODS AND RESULTS:
This review summarizes the results that have been published on plasma triglyceride, apolipoprotein B, HDL-cholesterol and LDL-cholesterol levels in humans and animal models in response to proanthocyanidin extracts and proanthocyanidin-rich foods. The physiological processes and biochemical pathways that are related to lipid homeostasis and affected by proanthocyanidin consumption are also discussed.
CONCLUSIONS:
Intestinal lipid absorption, chylomicron secretion by the intestine and VLDL secretion by the liver are the processes that are most repressed by Proanthocyanidins, which, therefore, induce hypolipidemic effects.
Int J Cardiol. 2007 Feb 14;115(3):326-33.
Cardioprotective effect of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats.[Pubmed:
16828181 ]
To investigate whether grape seed Proanthocyanidins (GSP) might protect the heart against myocardial injury (MI) induced by isoproterenol (ISO), in a rat model.
METHODS AND RESULTS:
GSP was administered orally to Wistar albino rats (150-180 g) in three different doses, by gastric gavage (50, 100 and 150 mg kg(-1) GSP), 6 days a week for 5 weeks. At the end of this period, all the rats, except the normal untreated rats that served as the control group, were administered ISO, 85 mg kg(-1) subcutaneously, for 2 consecutive days to induce myocardial injury. After 48 h, rats (n=6 per group) were anaesthetized with anesthetic ether, sacrificed and the levels of biochemical and histological observations of the heart tissues were performed.
Our results suggest that prior administration of GSP maintained the levels of the marker enzymes (AST, ALT, LDH and CK) in all the treatment groups (GSP-50-ISO, GSP-100-ISO and GSP-150-ISO) when compared to ISO-injected rats. The entire baseline groups also showed no significant alterations in serum marker enzyme levels in comparison to that of control group. Interestingly, in this study, there was no significant change in the basal levels of myocardial TBARS, GST, SOD and CAT on administration of GSP in all the three dosages (GSP-50-BL, GSP-100-BL and GSP-150-BL). However, a significant decrease occurred in the levels of GSH and GPx in group GSP-50-BL, which in the absence of any cellular injury (as evidenced by histological studies), is considered to be non-lethal. In the ISO-injected group there was a significant rise in TBARS and a significant decrease in GSH, GPx, GST, SOD and CAT when compared to group control. The administration of GSP maintained the activities of these enzymes close to normal levels when compared to group ISO, which proves the stress stabilizing action of GSP. The biochemical and histological evidence from this study shows that 100 and 150 mg kg(-1) of GSP protected against ISO-induced myocardial injury.
CONCLUSIONS:
This study demonstrates that GSP has a significant effect in the protection of heart against MI induced by ISO. We believe that pretreatment with GSP may contribute to developing novel strategies in the prevention and treatment of cardiotoxic effects of elevated levels of catecholamine.
Acta Pharmacol Sin. 2001 Dec;22(12):1117-20.
Anti-inflammatory effect and mechanism of proanthocyanidins from grape seeds.[Pubmed:
11749811]
To investigate the anti-inflammatory effect and mechanism of Proanthocyanidins (PA) from grape seeds.
METHODS AND RESULTS:
Croton oil-induced ear swelling in mice and carrageenan-induced hind paw edema in rats were prepared. The nitric oxide synthase (NOS) activity was measured by NADPH-diaphoras stain assay, nitric oxide (NO) content by Griess diazotization assay, N-acetyl-beta- D-glucosaminidase (beta-NAG) activity by spectrophotography, malondialdehyde (MDA) content by thiobarbituric acid (TBA) fluorescence technique, and IL-1beta, TNFalpha, and PGE2 content by radioimmunoassay (RIA).
PA 10-40 mg/kg ip inhibited carrageenan-induced paw edema in rats and croton oil-induced ear swelling in mice in a dose-dependent manner. PA 10 mg/kg reduced MDA content in inflamed paws, inhibited beta-NAG and NOS activity, and lowered the content of NO, IL-1beta, TNFalpha, and PGE2 in exudate from edema paws of rats induced by carrageenan. The inhibitory effect of PA on all above indices was more evident than that of dexamethasone 2 mg/kg.
CONCLUSIONS:
PA has anti-inflammatory effect on experimental inflammation in rats and mice. Its mechanisms of anti-inflammatory action are relevant to oxygen free radical scavenging, anti-lipid peroxidation, and inhibition of the formation of inflammatory cytokines.
Pharm Res. 2010 Jun;27(6):1092-102.
Dietary grape seed proanthocyanidins inhibit UVB-induced cyclooxygenase-2 expression and other inflammatory mediators in UVB-exposed skin and skin tumors of SKH-1 hairless mice.[Pubmed:
20143255]
The purpose of this study was to determine the chemopreventive mechanism of dietary grape seed Proanthocyanidins (GSPs) against ultraviolet (UV) radiation-induced skin tumor development in mice.
METHODS AND RESULTS:
Six-to-seven-week-old SKH-1 hairless mice were subjected to photocarcinogenesis protocol, and exposed to UVB radiation (180 mJ/cm(2)) three times/week for 24 weeks. Mice were fed a standard AIN76A control diet with or without supplementation with grape seed Proanthocyanidins (GSPs; 0.2% or 0.5%, w/w). At the termination of the experiment, mice were sacrificed, and skin and skin tumor samples were harvested and subjected to the analysis of biomarkers related to inflammation using immunostaining, western blot analysis, ELISA and real-time PCR.
Dietary GSPs inhibited UVB-induced infiltration of proinflammatory leukocytes and the levels of myeloperoxidase, cyclooxygenase-2 (COX-2), prostaglandin (PG) E(2), cyclin D1 and proliferating cell nuclear antigen (PCNA) in the skin and skin tumors compared to non-GSPs-treated UVB irradiated mouse skin and skin tumors. GSPs also significantly inhibited the levels of proinflammatory cytokines, tumor necrosis factor-alpha (P < 0.01), IL-1beta (P < 0.001) and IL-6 (P < 0.001), in UVB-exposed skin and skin tumors.
CONCLUSIONS:
The results from this study clearly suggest that dietary GSPs inhibit photocarcinogenesis in mice through the inhibition of UVB-induced inflammation and mediators of inflammation in mouse skin.