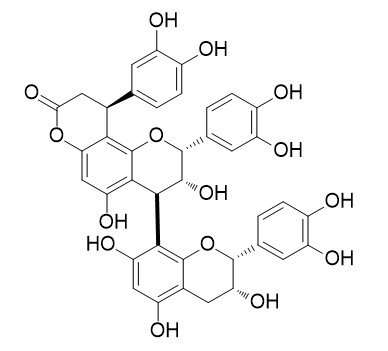
Cinchonain IIb
Cinchonain IIb shows high radical scavenging activity and reducing power.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Mol Med Rep.2023 Oct;28(4):193.
J Anal Methods Chem.2024, 2024:7703951.
Oncol Rep.2021, 46(2):166.
Int J Mol Sci.2019, 20(23):E6071
J Insect Sci.2020, 20(5):18.
Kor.J.Herbol.2024, 39(3):23-35
Food Science and Biotechnology2022, 10.1007.
Int J Biol Macromol.2025, 292:139225.
Pathogens.2018, 7(3):E62
Chulalongkorn University2024, 4761190
Related and Featured Products
Journal of the Brazilian Chemical Society, 2011, 22(11):2087-2093.
Phenylpropanoid Substituted Flavan-3-ols from Trichilia catigua and their In Vitro Antioxidative Activity[Reference:
WebLink]
METHODS AND RESULTS:
The new phenylpropanoid substituted flavan-3-ol apocynin E, together with eight known compounds, epicatechin, procyanidin B2, procyanidin B4, procyanidin C1, cinchonain Ia, cinchonain Ib, Cinchonain IIb, and Cinchonain IIa were isolated from an acetone-H2O extract of the air-dried stem bark of Trichilia catigua. The cinchonain Ia e Ib were reevaluated to its estereochemistry. All the compounds were characterized by spectroscopic analysis including 1D and 2D nuclear magnetic resonance (NMR) and mass spectrometry (MS) of their peracetate derivatives. The absolute configuration of the phenylpropanoid moiety was determined by circular dichroism (CD) spectra and by analyzing the anisotropic effects in the Dreiding model and nuclear Overhauser effect (NOESY NMR) experiments.
CONCLUSIONS:
The nine isolated compounds showed higher radical scavenging activity and reducing power than ascorbic acid and Trolox in the free-radical 2,2-diphenyl-1-picrylhydrazyl and Fe3+-Fe2+ reduction assay systems.
Chemical & Pharmaceutical Bulletin, 2008, 33(8):3142-3152.
Tannins and Related Compounds. XXXI. Isolation and Characterization of Proanthocyanidins in Kandelia candel (L.) DRUCE[Reference:
WebLink]
METHODS AND RESULTS:
Together with propelargonidin dimers (14, 15 and 16), and procyanidin trimers (23, 24 and 25) of common types, two novel proanthocyanidin dimers, kandelins A-1 (12) and A-2 (13), and four trimers, kandelins B-1 (19), B-2 (20), B-3 (21) and B-4 (22), which all contain a phenylpropanoid substituent in the upper flavan unit, have been isolated from the bark of Kandelia candel (L.) DRUCE (Rhizophoraceae). Spectroscopic evidence combined with chemical studies involving acid-catalyzed thiolytic degradation permitted the assignment of their structures.
CONCLUSIONS:
The presence in this plant source of flavan-3-ols, (+)-afzelechin (2), (+)-catechin (3), (-)-epicatechin (4) and (+)-gallocatechin (5), known proanthocyanidins B-1 (8), B-2 (9), C-1 (17) and trimer (18), and cinchonain Ia (6), cinchonain Ib (7), cinchonain IIa (10) and Cinchonain IIb (11) was also demonstrated.