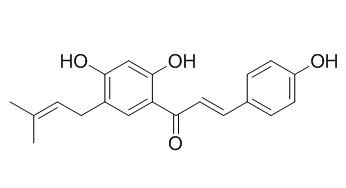
Bavachalcone
Bavachalcone has antibiotic or anticancer activities, it may be useful as a therapeutic drug for bone resorption-associated diseases.Bavachalcone can protect the endothelial function by increasing AMPK activity and MnSOD expression and reducing mitochondrial oxidative stress.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2023, 28(8):3376.
Chin Med.2022, 17(1):66.
J Chromatogr B Analyt Technol Biomed Life Sci.2021, 1187:123012.
Molecules.2023, 28(4):1785.
Sci. Rep.2015, 14-23
J Nat Med.2021, doi: 10.1007.
Molecules.2024, 29(3):671.
Phytofrontiers2024, 2690-5442.
Int J Mol Sci. 2014, 15(5):8443-57
BMB Rep.2018, 51(5):249-254
Related and Featured Products
Evid Based Complement Alternat Med. 2014;2014:958937.
Comparison of the Inhibitory Potential of Bavachalcone and Corylin against UDP-Glucuronosyltransferases.[Pubmed:
24829606]
Bavachalcone and corylin are two major bioactive compounds isolated from Psoralea corylifolia L., which has been widely used as traditional Chinese medicine for many years. As two antibiotic or anticancer drugs, Bavachalcone and corylin are used in combination with other drugs; thus it is necessary to evaluate potential pharmacokinetic herb-drug interactions (HDI) of the two bioactive compounds.
METHODS AND RESULTS:
The aim of the present study was to compare the effects of liver UDP-glucuronosyltransferase (UGT) 1A1, UGT1A3, UGT1A7, UGT1A8, UGT 1A10, and UGT2B4 inhibited by Bavachalcone and corylin. 4-Methylumbelliferone (4-MU) was used as a nonspecific "probe" substrate. Bavachalcone had stronger inhibition on UGT1A1 and UGT1A7 than corylin which did not inhibit UGT1A1, UGT1A3, UGT1A7, UGT1A8, UGT1A10, and UGT2B4. Data fitting using Dixon and Lineweaver-Burk plots demonstrated the noncompetitive inhibition of Bavachalcone against UGT1A1 and UGT1A7-mediated 4-MU glucuronidation reaction. The values of inhibition kinetic parameters (Ki) were 5.41 μ M and 4.51 μ M for UGT1A1 and UGT1A7, respectively.
CONCLUSIONS:
The results of present study suggested that there was a possibility of UGT1A1 and UGT1A7 inhibition-based herb-drug interaction associated with Bavachalcone and provided the basis for further in vivo studies to investigate the HDI potential between Bavachalcone and UGT substrates.
Biochem Pharmacol. 2008 Jun 1;75(11):2175-82.
Bavachalcone inhibits osteoclast differentiation through suppression of NFATc1 induction by RANKL.[Pubmed:
18433733]
Osteoclasts are cells that have a specialized role for bone resorption and are responsible for many bone diseases such as osteoporosis. As herbal products are invaluable sources in discovery of compounds for new therapies, we sought to identify compounds efficacious in suppressing osteoclastogenesis from medicinal plants that have been implicated for treatment of osteoporotic conditions.
METHODS AND RESULTS:
Bavachalcone was isolated from Psoralea corylifolia, and its effects on osteoclast differentiation were evaluated with primary cultures of osteoclast precursor cells. In addition, the molecular mechanism of action was investigated. Bavachalcone inhibited osteoclast formation from precursor cells with the IC(50) of approximately 1.5 microg ml(-1). The activation of MEK, ERK, and Akt by receptor activator of nuclear factor kappaB ligand (RANKL), the osteoclast differentiation factor, was prominently reduced in the presence of Bavachalcone. The induction of c-Fos and NFATc1, key transcription factors for osteoclastogenesis, by RANKL was also suppressed by Bavachalcone. In conclusion, Bavachalcone inhibits osteoclastogenesis by interfering with the ERK and Akt signaling pathways and the induction of c-Fos and NFATc1 during differentiation.
CONCLUSIONS:
Our results suggest that Bavachalcone may be useful as a therapeutic drug for bone resorption-associated diseases.
Pharmacology. 2015;95(3-4):105-10.
Bavachalcone-induced manganese superoxide dismutase expression through the AMP-activated protein kinase pathway in human endothelial cells.[Pubmed:
25766656]
Mitochondrial oxidative stress has been suggested as a major etiological factor in cardiovascular diseases. Manganese superoxide dismutase (MnSOD) is an essential antioxidant mitochondrial enzyme. Although polyphenols can induce MnSOD expression, their mechanism of action remains unclear.
METHODS AND RESULTS:
We examined the effect of Bavachalcone, a bioactive compound isolated from Psoralea corylifolia, on MnSOD protein expression and explored whether this effect is mediated through the AMP-activated protein kinase (AMPK) signaling pathway. Our data showed that Bavachalcone enhanced the luciferase activity of the MnSOD promoter and increased MnSOD mRNA and protein expressions. Moreover, Bavachalcone suppressed the mitochondrial superoxide production in endothelial cells. Conversely, Bavachalcone stimulated liver kinase B1 and AMPKα phosphorylation. mRNA interference by using short hairpin RNA (shRNA) of AMPK inhibited Bavachalcone-induced MnSOD expression. A-769662, an AMPK activator, also stimulated AMPK activity and increased MnSOD expression. Furthermore, AMPK knockdown by shRNA-AMPK reversed the inhibitory effects of Bavachalcone on mitochondrial superoxide production in endothelial cells.
CONCLUSIONS:
These findings indicate that Bavachalcone can protect the endothelial function by increasing AMPK activity and MnSOD expression and reducing mitochondrial oxidative stress.