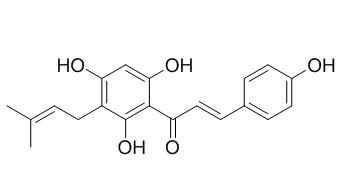
Desmethylxanthohumol
Desmethylxanthohumol shows estrogenic activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Sci Rep.2018, 8(1):12970
Int J Biol Macromol.2025, 292:139225.
Environ Toxicol.2021, 36(9):1848-1856.
Univerzita Karlova2021, 20.500.11956.
Nat Prod Commun.2017, 12(5):771-778
Curr Eye Res.2018, 43(1):27-34
Korean Journal of Pharmacognosy2018, 49(3):270-277
Chem Biol Interact.2016, 260:168-175
Front Nutr.2021, 8: 687851.
Evidence-based Compl.&Alternative Med.2023, 5417813
Related and Featured Products
Planta Medica, 2009, 75(07):757-762.
Dynamic Residual Complexity of Natural Products by qHNMR: Solution Stability of Desmethylxanthohumol.[Reference:
WebLink]
The use of chromatographic assays to assess the residual complexity of materials that are purified from natural sources by chromatographic means is, in a sense, a case of the fox watching the henhouse. Beside their static residual complexity, which is intrinsic to their metabolic origin, biologically active natural materials can also be involved in chemical reactions that lead to dynamic residual complexity.
METHODS AND RESULTS:
The present study examines the dynamics of the hop prenylphenol, Desmethylxanthohumol (DMX), by means of quantitative ¹H-NMR (qHNMR) in a setting that mimics in vitro and physiological conditions. The experiments provide a comprehensive, time-resolved, and mechanistic picture of the spontaneous isomerization of DMX into congeneric flavanones, including their ¹H/²D isotopomers. Formation of the potent phytoestrogen, 8-prenylnaringenin (8PN), suggests that measurable estrogenic activity even of high-purity DMX is an artifact. Together with previously established qHNMR assays including purity activity relationships (PARs), dynamic qHNMR assays complement important steps of the post-isolation evaluation of natural products.
CONCLUSIONS:
Thus, qHNMR allows assessment of several unexpected effects that potentially break the assumed linkage between a single chemical entity (SCE) and biological endpoints.
Eur J Med Chem . 2017 Jan 5;125:335-345.
Synthesis and antioxidant evaluation of desmethylxanthohumol analogs and their dimers[Pubmed:
27688188]
Abstract
Four ring-closed analogs of natural prenylated chalcone Desmethylxanthohumol (1) and their dimers were synthesized from the commercially available 1-(2,4,6-trihydroxyphenyl)ethan-1-one in five and six linear steps, respectively. The structures of the eight new derivatives were confirmed using1H NMR, 13C NMR and HRMS. The antioxidant activity of the new chalcone derivatives were evaluated in a PC12 cell model of H2O2-induced oxidative damage. The SAR studies suggested that the catechol motif was essential for the antioxidant activity. Moreover, the dimers showed better antioxidant activity than their corresponding monomers did. Among them, compound 14d was the most potent and increased PC12 cell viability from 25% to 85%. Flow cytometric analysis showed that compound 14d, the most potent compound, decreased the apoptotic PC12 cell percentage and significantly reduced the LDH release and 8-OHdG generation but increased the GSH levels in H2O2-treated PC12 cells. Furthermore, compound 14d had a higher FRAP value than that of gallic acid. It also reduced the stable ABTS+ free radical with a lower EC50 than that of gallic acid.
Keywords: Antioxidant; Chalcone; Desmethylxanthohumol; Dimer; Synthesis.
Journal of Agricultural and Food Chemistry, 2007, 55(1):61-66.
Relevance of organic farming and effect of climatological conditions on the formation of alpha-acids, beta-acids, desmethylxanthohumol, and xanthohumol in hop (Humulus lupulus L.).[Reference:
WebLink]
The concentrations of alpha-acids, beta-acids, Desmethylxanthohumol, and xanthohumol were monitored in the hop varieties Admiral (A), Wye Challenger (WC), and First Gold (FG) during the harvest seasons of 2003 through 2005.
METHODS AND RESULTS:
Hops grown under an organic regimen were compared to plants grown conventionally in hop fields in close vicinity. The concentrations of the key compounds depended very much on climatological conditions showing, in general, highest levels in poorest weather conditions (2004). Of the three varieties studied, FG was the only one showing a clear trend for higher concentrations of secondary metabolites under organic growing conditions than under conventional farming conditions. Cultivation of A and WC seems to be very sensitive to climatic conditions and environmental stresses caused by pests and diseases, thereby leading to various results.
CONCLUSIONS:
WC proved to be a rich source of bioactive chalcones, particularly Desmethylxanthohumol.