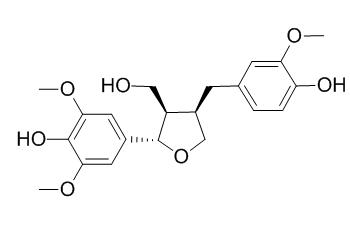
5'-Methoxylariciresinol
5'-Methoxylariciresinol is a natural product from Stellera chamaejasme.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Heliyon.2024, 10(23):e40758.
Sci Rep.2019, 9:12132
Industrial Crops and Products2018, 353-362
Front Aging Neurosci.2019, 11:230
Biomolecules.2024, 14(5):589.
Int J Mol Sci.2018, 19(9):E2681
Appl Microbiol Biotechnol.2016, 100(9):3965-77
J of the Korean Society of Food Science and Nutrition2016, 45(7):1017-1025
Phytochem Anal.2021, 32(6):970-981.
J Biotechnol.2020, 318:10-19.
Related and Featured Products
Zhongguo Zhong Yao Za Zhi. 2011 Dec;36(24):3457-62.
Studies on chemical constitutes from callus cultures of Stellera chamaejasme.[Pubmed:
22368856]
METHODS AND RESULTS:
From callus cultures of Stellera chamaejasme, 17 compounds were isolated. Based on their physical and chemical data and spectroscopic analysis, they were identified as syringaresinol (1), medioresinol (2), pinoresinol (3), (1R, 2S, 5R, 6S)- 2-(4- hydroxyphenyl)-6-(3-methoxy-4-hydroxyphenyl)-3, 7-dioxabicyclo [3, 3, 0] octane (4), epipinoresinol (5), caruilignan D (6), 3-oxo-guai-4-ene-11, 12-diol (7), (-) -lariciresinol (8), tetrahydro-2-(4-hydroxy-3-methoxyphenyl)-4-[(4-hydroxyphenyl) methyl]-3-furanmethanol (9), 5'-Methoxylariciresinol (10), vladinol D (11), cyclo (L-Pro-L-Val) (12), oxomatairesinol (13), (+) -guayarol (14); acutissimalignan B (15), isolariciresinol (16), and beta-sitosterol (17), respectively.
CONCLUSIONS:
Among these compounds, 12 was a cyclodipeptide, 7 was a sesquiterpene, and the others except 17 were lignans. All compounds were first isolated from callus cultures of S. chamaejasme.
Zhongguo Zhong Yao Za Zhi. 2012 May;37(9):1241-4.
Non-alkaloid chemical constituents from Coptis chinensis.[Pubmed:
22803368]
To separate and identify chemical constituents from Coptis chinensis.
METHODS AND RESULTS:
The compounds were separated and purified by various chromatographic techniques. Their structures were identified on the basis of their physicochemical properties using spectral techniques such as NMR and MS. Thirteen compounds were separated from ethanol extracts of C. chinensis, including seven lignans, three simple phenylpropanoids, two flavones and one phenolic acid, and identified as erythro-guaiacylglycerol-8-O-4'-(coniferyl alcohol) ether (1), threo-guaiacylglycerol-8-O-4'-(coniferyl alcohol) ether (2), (+)-pinoresinol (3), (+)-medioresinol (4), (+)-lariciresinol (5), (+)-5'-Methoxylariciresinol (6), (+)-isolariciresinol (7), chlorogenic acid (8), ferulic acid (9), Z-octadecyl caffeate (10), rhamnetin (11), wogonin (12), and vanillic acid (13).
CONCLUSIONS:
Compounds 1, 2, 4, 6, 10-13 were separated from the genus Coptis for the first time.
3,3',4',5,6,7,8-heptamethoxyflavone
Catalog No: CFN95021
CAS No: 1178-24-1
Price: $268/10mg
Poricoic acid G
Catalog No: CFN95056
CAS No: 415724-84-4
Price: $318/5mg
3,7,23,24-tetrahydroxycucurbita-5,25-dien-19-al
Catalog No: CFN95168
CAS No: 1446447-97-7
Price: $318/5mg
Polygalin J
Catalog No: CFN95175
CAS No: N/A
Price: $318/5mg
Gypenoside XIII
Catalog No: CFN95239
CAS No: 80325-22-0
Price: $288/10mg
15-Deoxypulic acid
Catalog No: CFN95262
CAS No: 95523-05-0
Price: $368/5mg
Deoxy euphorbia factor L1
Catalog No: CFN95340
CAS No: 247099-01-0
Price: $318/10mg
10-Methoxygambogenic acid
Catalog No: CFN95450
CAS No: 2095102-72-8
Price: $318/10mg
8-Epiloganin
Catalog No: CFN95478
CAS No: 79172-04-6
Price: $318/20mg
Methyl nomilinate
Catalog No: CFN95576
CAS No: 77887-51-5
Price: $318/5mg