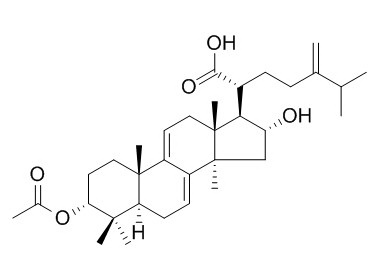
3-Epidehydropachymic acid
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J. of Agricultural Science2015, 1916-9760
The Journal of Korean Medicine2023, 44(4):26-40.
J Chromatogr A.2024, 1714:464544.
BioResources J.2020, 15(3).
Molecules2022, 27(14),4462
Applied Biological Chemistry 2021, 64(75)
Phytomedicine.2015, 22(11):1027-36
Integr Cancer Ther.2018, 17(3):832-843
Biomedicines.2022, 10(3):583.
Chem Biodivers.2023, 20(10):e202300741.
Related and Featured Products
Separation Science and Technology, 2014, 49(17):2765-2771.
Acid-Alkali Extraction ofTriterpene Acids from Poria and Preparative Separation by High-Speed Counter-Current Chromatography.[Reference:
WebLink]
METHODS AND RESULTS:
A method for the acid-alkali extraction and preparative separation of triterpene acids from poria was established. The triterpene acids were enriched and separated into two fractions after extraction at the optimized pH value. The two fractions were subjected to high-speed counter-current chromatography for the preparative separation of triterpene acids, separately.
CONCLUSIONS:
As a result, dehydropachymic acid, pachymic acid, 3-Epidehydropachymic acid, poricoic acid B, dehydrotumulosic acid, and 3-epi-dehydrotumulosic acid were obtained with purities of 94.1%, 96.2%, 93.5%, 85.9%, 80.1%, and 93.1%, respectively. The structures were identified by ESI-MS, 1H NMR, and 13C NMR.
Licoarylcoumarin
Catalog No: CFN95072
CAS No: 125709-31-1
Price: $413/5mg
Methyl caffeate acid
Catalog No: CFN95561
CAS No: 3843-74-1
Price: Inquiry(manager@chemfaces.com)
Rhamnocitrin 3-apiosyl-(1->2)-glucoside
Catalog No: CFN95145
CAS No: 148031-68-9
Price: $318/10mg
(3S,5S,E)-1,7-Diphenylhept-1-ene-3,5-diol
Catalog No: CFN95195
CAS No: 87095-75-8
Price: $318/5mg
Quercetin 7-O-(6''-O-malonyl)-beta-D-glucoside
Catalog No: CFN95208
CAS No: 98767-37-4
Price: $388/10mg
Sphenanlignan
Catalog No: CFN95245
CAS No: 866347-36-6
Price: $318/5mg
Nortrachelogenin-5'-C-beta-glucoside
Catalog No: CFN95251
CAS No: 858127-39-6
Price: $318/10mg
5,6,7,3',4',5'-Hexamethoxyflavanone
Catalog No: CFN95376
CAS No: 74064-17-8
Price: $318/10mg
11beta,13-Dihydrolactucin
Catalog No: CFN95433
CAS No: 83117-63-9
Price: $318/10mg
10-Methoxygambogenic acid
Catalog No: CFN95450
CAS No: 2095102-72-8
Price: $318/10mg