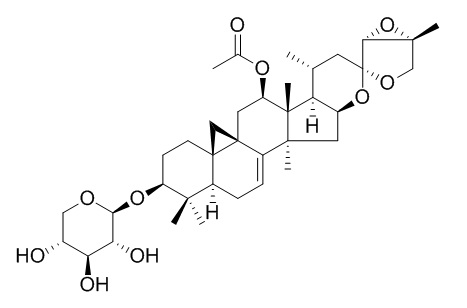
26-Deoxycimicifugoside
26-Deoxycimicifugoside is a natural product from Cimicifuga foetida L.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biorxiv2019, 10.1101
Univerzita Karlova2021, 20.500.11956.
Mol Cell.2017, 68(4):673-685
Front Chem.2023, 11:1245071.
Asian Journal of Chemistry2014, 26(22):7811-7816
Molecules.2018, 23(9):E2121
Food Chem.2018, 252:207-214
Plant Archives2020, 2(1),2929-2934
Korean Herb. Med. Inf. 2016, 4(1):35-42
Anticancer Res.2022, 42(9):4403-4410.
Related and Featured Products
Periodical of Ocean University of China, 2014 , 44 (11) :74-80.
Cycloartane Triterpenoid Saponins from Cimicifuga foetida and Their Cytotoxic Activity[Reference:
WebLink]
METHODS AND RESULTS:
Fifteen cycloartane saponins(1-15)were isolated from EtOAc fraction of EtOH extracts of the C.foetidarhizoma using silicon chromatography methods.And comparison their spectroscopic data with those of literatures allowed these compounds to be identified as 25-anhydrocimigenol-3-O-β-D-xylopyranoside(1),25-O-acetyl-cimigenol-3-O-β-D-xylopyranoside(2),25-O-acetyl-7,8-didehydrocimigenol-3-O-β-D-xylopyranoside(3),25-O-acetyl-cimigenol-3-O-α-L-arabinopyranoside(4),asiaticoside A(5),asiaticoside B(6),isocimipodocarpaside(7),cimicidanol(8),26-Deoxyactein(9),cimigenol-3-O-β-D-xylopyranoside(10),24-O-acetylisodahurinol-3-O-β-D-xylopyranoside(11),23-O-acetylshengmanol-3-O-β-D-xylopyranoside(12),26-Deoxycimicifugoside(13),cimicifugoside H-1(14),and cimicifugoside H-2(15).
CONCLUSIONS:
Compound 6 was isolated from this genus for the first time,and compounds 3,7and 11 were firstly isolated from the species.
Additionally,compounds 2-6 showed potent cytotoxicities against the selected HeLa and MCF-7cancer cell lines with IC50 values ranging from 7.25-23.61μM.The present research further enriches chemodiveristy of the traditional medicine of C.foetida,and provides the potential structure activity relationship.
Spectrochim Acta A Mol Biomol Spectrosc. 2012 Jul;93:10-8.
One new and six known triterpene xylosides from Cimicifuga racemosa: FT-IR, Raman and NMR studies and DFT calculations.[Pubmed:
22465763 ]
One new and six known triterpene xylosides were isolated from Cimicifuga racemosa (black cohosh, Actaea racemosa).
METHODS AND RESULTS:
The structure of a new compound, designated as isocimipodocarpaside (1), was established to be (24S)-3β-hydroxy-24,25-oxiirane-16,23-dione-9,10-seco-9,19-cyclolanost-1(10),7(8),9(11)-trien 3-O-β-d-xylopyranoside, by means of (1)H and (13)C NMR, IR and Raman spectroscopies and Mass Spectrometry. The six known compounds are: 23-epi-26-Deoxycimicifugoside (2), 23-epi-26-deoxyactein (3), 25-anhydrocimigenol xyloside (4), 23-O-acetylshengmanol xyloside (5), 25-O-acetylcimigenol xyloside (6) and 3'-O-acetylcimicifugoside H-1 (7). On the basis of NMR data supported by DFT calculations of NMR shielding constants of (2), its structure, previously described as 26-Deoxycimicifugoside was corrected and determined as 23-epi-26-Deoxycimicifugoside.
CONCLUSIONS:
The (13)C CPMAS NMR spectra of the studied compounds (1)-(7) provided data on their solid-state interactions. The IR and Raman spectra in the CO, CC, and CH stretching vibration regions clearly discriminate different triterpenes found in C. racemosa.