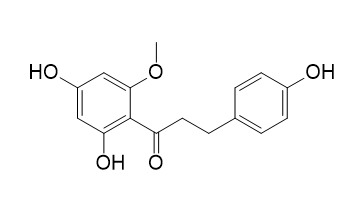
2'-O-Methylphloretin (4,2',4'-Trihydroxy-6'-methoxydihydrochalcone)
2′-O-Methylphloretin inhibits the tumor cell line replication.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Synthetic and Systems Biotechnology2023, j.synbio.
Front Plant Sci.2022, 13:982771.
BMC Complement Altern Med.2017, 17(1):393
Biomolecules.2023, 13(2):227.
Int J Mol Med.2016, 37(2):501-8
Environ Toxicol.2024, 39(5):2927-2936.
Molecules.2023, 28(4):1785.
Journal of Medical Sciences2024, 44(5):p 222-227.
Analytical Methods2018, 10(27)
Molecules.2019, 24(11):E2044
Related and Featured Products
J Agric Food Chem . 2003 May 21;51(11):3309-3312.
Dihydrochalcones: evaluation as novel radical scavenging antioxidants[Pubmed:
12744659]
Dihydrochalcones are a family of bicyclic flavonoids, defined by the presence of two benzene rings joined by a saturated three carbon bridge. In the present study, we systematically examined the antioxidant activities of dihydrochalcones against the stable free radical (1,1-diphenyl-2-picrylhydrazyl) and lipid peroxidation in the erythrocyte membrane. All dihydrochalcones exhibited higher antioxidant activities than the corresponding flavanones. The (1)H NMR analysis indicated that the active dihydrochalcone has a time-averaged conformation in which the aromatic A ring is orthogonal to the carbonyl group, while the inactive dihydrochalcone such as 2'-O-methyl-phloretin has a strongly hydrogen-bonded phenolic hydroxyl group, suggestive of a coplanar conformation. A hydroxyl group at the 2'-position of the dihydrochalcone A ring, newly formed by reduction of the flavanone C ring, is an essential pharmacophore for its radical scavenging potential.
Phytochemistry . 2000 Nov;55(5):439-446.
(Rel)-1beta,2alpha-di-(2,4-dihydroxy-6-methoxybenzoyl)-3beta, 4alpha-di-(4-methoxyphenyl)-cyclobutane and other flavonoids from the aerial parts of Goniothalamus gardneri and Goniothalamus thwaitesii[Pubmed:
11140605]
The aerial parts of Goniothalamus gardneri (Annonaceae) has yielded the known flavonoids 2'-hydroxy-4,4',6'-trimethoxychalcone (flavokawain A), 2',4'-dihydroxy-4,6'-dimethoxydihydrochalcone, 4,2',4'-trihydroxy-6'-methoxydihydrochalcone, 5,7,4'-trimethoxyflavanone (naringenin trimethyl ether) and 7-hydroxy-5,4'-dimethoxyflavanone (tsugafolin) together with three novel compounds, the dimer characterised as (rel)-1beta,2alpha-di-(2,4-dihydroxy-6-methoxybenzoyl)-3beta,4alpha-di-(4-methoxyphenyl)-cyclobutane, 2',4'-dihydroxy-4,6'-dimethoxychalcone and 2'-hydroxy-4,4',6'-trimethoxydihydrochalcone. The last two have previously been synthesised but appear to be new natural products. A similar study of the aerial parts of G. thwaitesii led only to the isolation of the known flavonoids myricetin 4'-O-methyl ether-3-O-alpha-L-rhamnopyranoside (mearnsitrin) and myricetin-3-O-methyl ether (annulatin), together with the triterpenes friedelinol, friedelin and betulinic acid. All compounds were identified by spectroscopic analysis and, for known compounds, by comparison with published data.