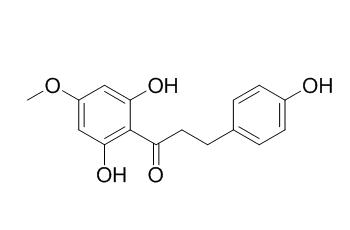
Asebogenin
Asebogenin has anti-bacterial activity, it shows inhibitory activity against S. aureus and methicillin-resistant S. aureus (IC50 of 10 and 4.5 micrograms/ml, respectively); it shows antiplasmodial activities against both a chloroquine-sensitive and a resistant strain of Plasmodium falciparum. Asebogenin can inhibit the proliferation of murine B cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Acta Edulis Fungi2020, 27(02):63-76.
Environ Toxicol.2021, doi: 10.1002
J Herbmed Pharmacol.2018, 7(4):280-286
J Cachexia Sarcopenia Muscle.2022, 13(6):3149-3162.
Biomedicine & Pharmacotherapy2022, 153:113404.
EXCLI J.2023, 22:482-498.
Eur J Pharm Sci.2016, 94:33-45
Appl. Sci.2020, 10(20),7374.
J Pharm Biomed Anal2016, 118:183-194
Mol Med Rep.2014, 9(5):1653-9
Related and Featured Products
2',6'-Dihydroxy 4',4-dimethoxydihydrochalcone
Catalog No: CFN70345
CAS No: 35241-54-4
Price: Inquiry(manager@chemfaces.com)
2'-O-Methylphloretin (4,2',4'-Trihydroxy-6'-methoxydihydrochalcone)
Catalog No: CFN91680
CAS No: 111316-17-7
Price: Inquiry(manager@chemfaces.com)
Helichrysetin
Catalog No: CFN97065
CAS No: 62014-87-3
Price: $268/5mg
2,4-Dihydroxy-4,6-dimethoxydihydrochalcone
Catalog No: CFN97962
CAS No: 75679-58-2
Price: Inquiry(manager@chemfaces.com)
2',6'-Dihydroxy-4,4'-dimethoxychalcone
Catalog No: CFN70304
CAS No: 20621-49-2
Price: Inquiry(manager@chemfaces.com)
4-O-Methylhelichrysetin
Catalog No: CFN96080
CAS No: 56121-44-9
Price: $413/5mg
2'-O-Methylhelichrysetin
Catalog No: CFN97920
CAS No: 123316-64-3
Price: Inquiry(manager@chemfaces.com)
Flavokawain C
Catalog No: CFN90741
CAS No: 56798-34-6
Price: $238/10mg
Flavokawain A
Catalog No: CFN98446
CAS No: 3420-72-2
Price: $238/10mg
2',4',6',4-Tetramethoxychalcone
Catalog No: CFN91683
CAS No: 94103-36-3
Price: Inquiry(manager@chemfaces.com)
J Ethnopharmacol. 2010 Apr 21;128(3):583-9.
Anti-secretory, anti-inflammatory and anti-Helicobacter pylori activities of several fractions isolated from Piper carpunya Ruiz & Pav.[Pubmed:
20152892]
METHODS AND RESULTS:
Eight fractions showed inhibition of MPO enzyme (F I-IV, X, XII, XIV and XV). The highest inhibition was observed with F XIV (50microg/mL, 60.9%, p<0.001). F X and XII were the most active ones, inhibiting the gastric H(+), K(+)-ATPase activity with IC(50) values equal to 22.3microg/mL and 28.1microg/mL, respectively. All fractions, except F XV, presented detectable anti-Helicobacter pylori activity, with a diameter of inhibition zones ranging from 11mm up to 50mm. The best anti-Helicobacter pylori activity was obtained with F III and V. Both fractions killed Helicobacter pylori with lowest concentration values, about 6.25mug/mL.
CONCLUSIONS:
Sixteen pure compounds were isolated, five of them are flavonoids that possess strong anti-oxidant and free radical scavenging activity, e.g. vitexin, isovitexin, and rhamnopyranosylvitexin.
Terpenoids like sitosterol, stigmasterol and phytol, which have shown gastroprotective activity, and dihydrochalcones, like Asebogenin, with anti-bacterial activity, were also isolated. Furthermore, the rare neolignan 1, that is a DNA polymerase beta lyase inhibitor, and (6S, 9S)-roseoside, that shows strong anti-bacterial activity, were isolated, for the first time, from the genus Piper.
Planta Med. 2001 Mar;67(2):186-8.
Dihydrochalcones from Piper longicaudatum.[Pubmed:
11301876 ]
METHODS AND RESULTS:
Bioactivity-guided fractionation of an ethanolic extract of the leaves and twigs of Piper longicaudatum Trelease & Yunker (Piperaceae) resulted in the isolation of one new (1) and three known (2-4) dihydrochalcones. The known compounds are: 2',6'-dihydroxy-4'-methoxydihydrochalcone (2), 2',6',4-trihydroxy-4'-methoxydihydrochalcone (Asebogenin) (3), and 2'-hydroxy-4'-methoxy-2'-[1-hydroxy-1-methylethyl]-2",3"-dihy- drofurano[4",5":5',6"]-3"-[2-hydroxy-5-methoxycarbonylphe- nyl]dihydrochalcone (piperaduncin B) (4). The new compound is 2'-hydroxy-4'-methoxy-2"-[2-hydroxy-5-methoxycarbonyl- phenyl]-furano[4",5":5',6']-dihydrochalcone (longicaudatin) (1).
CONCLUSIONS:
Compounds 1-4 were tested for antibacterial activity against S. aureus and methicillin-resistant S. aureus (MRSA); only compound 3 showed inhibitory activity (IC50 of 10 and 4.5 micrograms/ml, respectively).
Trop Med Int Health. 1999 Sep;4(9):611-5.
The in vitro antiplasmodial activities of 14 plant species traditionally used in Central America for the treatment of malaria or fever were evaluated. Lipophilic extracts of Piper hispidum, Siparuna andina, S. pauciflora, S. tonduziana, and Xylopia cf. fr[Pubmed:
10540301]
METHODS AND RESULTS:
The in vitro antiplasmodial activities of 14 plant species traditionally used in Central America for the treatment of malaria or fever were evaluated. Lipophilic extracts of Piper hispidum, Siparuna andina, S. pauciflora, S. tonduziana, and Xylopia cf. frutescens, proved to be active against both a chloroquine-sensitive and a resistant strain of Plasmodium falciparum. IC50 values ranged between 3.0 microg/ml and 21.9 microg/ml; however, moderate cytotoxicity of active extracts was observed.
CONCLUSIONS:
Bioactivity-guided fractionation of Piper hispidum yielded 2',4, 6'-trihydroxy-4'-methoxydihydrochalcone (Asebogenin) as an active compound.
J Nat Prod. 2005 Mar;68(3):392-6.
Dihydrochalcones from the leaves of Pieris japonica.[Pubmed:
15787442 ]
METHODS AND RESULTS:
Six new dihydrochalcones, 3-hydroxyasebotin (5), Asebogenin 2'-O-beta-D-ribohexo-3-ulopyranoside (6), 2' '-acetylasebotin (7), 3',4,5'-trihydroxy-4'-methoxydihydrochalcone 3',5'-di-O-beta-D-glucopyranoside (8), and pierotins A (9) and B (10), along with four known dihydrochalcones, phloretin (1), phlorizin (2), Asebogenin (3), and asebotin (4), were isolated from the leaves of Pieris japonica. Their structures were elucidated on the basis of spectroscopic analysis including HMQC, HMBC, NOESY, and X-ray crystal diffraction.
CONCLUSIONS:
Compounds 1, 3-5, and 7-10 inhibited the proliferation of murine B cells and compounds 5 and 10 inhibited the proliferation of murine T cells in vitro significantly.
3,3',4',5,6,7,8-heptamethoxyflavone
Catalog No: CFN95021
CAS No: 1178-24-1
Price: $268/10mg
Isololiolide
Catalog No: CFN95106
CAS No: 38274-00-9
Price: $318/5mg
(3E,5E,11E)-tridecatriene-7,9-diyne-1,2-diacetate
Catalog No: CFN95191
CAS No: 94753-06-7
Price: $318/5mg
Prionanthoside
Catalog No: CFN95206
CAS No: 161842-81-5
Price: $318/10mg
Vaccarin E
Catalog No: CFN95252
CAS No: 2252345-81-4
Price: $318/10mg
11-Deoxyisomogroside V
Catalog No: CFN95288
CAS No: 1628293-32-2
Price: $368/10mg
Gynosaponin TN2
Catalog No: CFN95331
CAS No: 77658-95-8
Price: $318/5mg
Elgonica dimer A
Catalog No: CFN95445
CAS No: 132210-48-1
Price: $368/5mg
Oxytroflavoside B
Catalog No: CFN95490
CAS No: 1391144-81-2
Price: $318/10mg
Mahuannin E
Catalog No: CFN95585
CAS No: 1173887-70-1
Price: $318/5mg