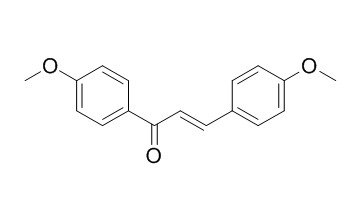
4,4'-Dimethoxychalcone
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Korean Herb. Med. Inf. 2016, 4(1):35-42
Arch Biochem Biophys.2020, 687:108384.
Plant Physiol Biochem.2023, 201:107795.
J Med Food.2020, 23(6):633-640.
Front Microbiol.2022, 13:835463.
BMC Plant Biol.2020, 20(1):214.
Chemistry of Plant Raw Materials2022, 20220210569.
Molecules.2023, 28(4):1785.
J Nat Prod.2021, 84(9):2544-2553.
Food Bioscience2024, 58:103691.
Related and Featured Products
Research on Chemical Intermediates, 2017, 43(1):73-89.
The anodic reactivity of 4,4′-dimethoxychalcone: a synthetic and mechanistic investigation。[Reference:
WebLink]
METHODS AND RESULTS:
The anodic oxidation of the 4,4′-dimethoxychalcone (DMC) was investigated by different electrochemical methods at a platinum working electrode and in acetonitrile as a solvent. The DMC exhibited a single irreversible anodic peak around 1.6 V versus Ag/AgCl. On the time scale of cyclic voltammetry experiments, the highly reactive radical cation issued from the first electron transfer underwent a second order rate-limiting reaction. The potential imposed electrolyses of DMC led to the formation of a semi-conducting oligomer with 40 % yield.
CONCLUSIONS:
Using different physico-chemicals methods, the structural study confirmed the formation of an o-phenylenevinylene oligomer.
The values of the corresponding optical and electrochemical band gaps were calculated to be 3.15 and 2.86 eV, respectively. Finally, a mechanism for the DMC electro-oligomerization was proposed on the basis of the obtained results.