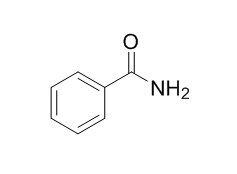
Benzamide
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Oncol Rep.2016, 35(3):1356-64
J Ethnopharmacol.2016, 192:370-381
Antioxidants (Basel).2024, 13(8):951.
J Neuroinflammation.2020, 17(1):75.
PLoS One.2017, 12(3):e0173585
Materials Today Communications2023, 37:107216
Molecular & Cellular Toxicology2022, 10.1007:s13273-022-00277-3
Foods.2021, 10(11):2627.
Phytochemistry.2017, 141:162-170
Separations2023, 10(2), 131.
Related and Featured Products
Inorg Chem. 2015 Apr 20;54(8):3958-69.
Density functional theory study of Rh(III)-catalyzed C-H activations and intermolecular annulations between benzamide derivatives and allenes.[Pubmed:
25856513]
METHODS AND RESULTS:
Density functional theory has been applied to gain insight into the Cp*Rh(OAc)2-catalyzed C-H activation and intermolecular annulation of Benzamide derivatives with allenes. The study shows that the reactions proceed in three steps: (1) C-H activation induced by Rh catalyst reacting with Benzamide derivatives, (2) carborhodation of allene, and (3) regeneration of Rh catalyst. The results indicate that the N-H deprotonation makes the following C-H activation much easier. The regio- and stereoselectivities of 1a (N-pivaloyloxy Benzamide)/2a (cyclohexylallene) and 1b (N-pivaloyloxy-4-methyl-Benzamide)/2b (1,1-dimethyl allene) depend on the allene carborhodation step. The steric hindrance effect is the dominant factor. We also discuss the reaction mechanism of 1c (N-methoxy Benzamide)/2a.
CONCLUSIONS:
The chemoselectivity between 1c/2a is determined by the N-O cleavage step. Replacement of OPiv by OMe leads to loss of the stabilization effect provided by C=O in OPiv. Additionally, Cp*Rh(OAc)(OPiv) is produced in the Cp*Rh(OAc)2 regeneration step, which can work as catalyst as well.