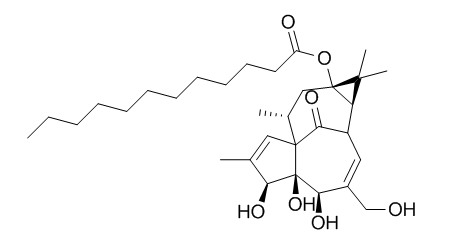
13-Oxyingenol dodecanoat
13-Oxyingenol dodecanoat shows moderate cytotoxicity with IC50 values being 35.59 ± 5.37 μmol·L-1 (Caco-2), 24.04 ± 4.70 μmol·L-1 (MCF-7), and 22.24 ± 5.19 μmol·L-1 (MCF-7/ADM).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytomedicine.2020, 79, 153351
Chem Biol Interact.2016, 258:59-68
Mol Med Rep.2015, 12(5):7789-95
Korean Journal of Pharmacognosy2018, 49(4):349-361
Expert Opin Ther Targets.2024, :1-11.
Biomed Pharmacother.2019, 116:108987
J Sci Food Agric.2023, 103(1):213-220.
Molecular & Cellular Toxicology2022, 10.1007:s13273-022-00277-3
Food Science and Biotechnology2022, 10.1007.
Asian Pac J Cancer Prev. 2020, 21(4):935-941.
Related and Featured Products
Chin J Nat Med. 2016 Dec;14(12):939-945.
Regio- and stereo-selective hydroxylations of ingenane diterpenoids by Mortierella ramanniana and Gibberella fujikuroi.[Pubmed:
28262122 ]
The regio- and stereo-selective hydroxylations of two ingenane diterpenoids, 20-deoxyingenol (1) and 13-Oxyingenol dodecanoat (2), by the filamentous fungi Mortierella ramanniana and Gibberella fujikuroi were investigated in the present study.
METHODS AND RESULTS:
Four undescribed metabolites (3-6) of substrate 1 and two undescribed metabolites (7 and 8) of substrate 2 were isolated. All the metabolites were identified as hydroxylated ingenane derivatives by extensive NMR and HR-ESI-MS data analyses. All the biotransformed compounds and the substrates were evaluated for their cytotoxicities against three human cancer cell lines, including human colon cancer Caco-2, breast cancer MCF-7, and adriamycin (ADM)-resistant MCF-7/ADM cell lines. All ingenane alcohols (1, and 3-6) displayed no significant cytotoxic activities. The substrate 13-Oxyingenol dodecanoat (2) showed moderate cytotoxicity with IC50 values being 35.59 ± 5.37 μmol·L-1 (Caco-2), 24.04 ± 4.70 μmol·L-1 (MCF-7), and 22.24 ± 5.19 μmol·L-1 (MCF-7/ADM). However, metabolites 7 and 8 displayed no significant cytotoxicity.
CONCLUSIONS:
These results indicated that the hydroxylation at the C-13 aliphatic acid ester of substrate 2 can significantly reduce the cytotoxic activity.