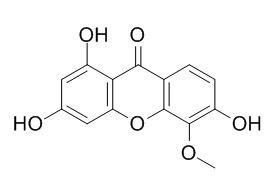
1,3,6-Trihydroxy-5-methoxyxanthone
Standard reference
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Breast Cancer.2015, 18(2):112-118
University of Stuttgart2021, 11682.
Am J Chin Med.2016, 44(6):1255-1271
Int J Mol Sci.2017, 19(1)
Oxid Med Cell Longev.2022, 2022:9139338.
J of Advanced Scientific R.2020, 11(3), p109-120.
Agronomy2020, 10(10),1489
Chem Biodivers.2023, 20(10):e202300741.
Mol Med Rep.2023 Oct;28(4):193.
Separations2023, 10(2), 131.
Related and Featured Products
Bellidifolin
Catalog No: CFN89132
CAS No: 2798-25-6
Price: $268/10mg
Gentisein
Catalog No: CFN92398
CAS No: 529-49-7
Price: Inquiry(manager@chemfaces.com)
1,3,5-Trihydroxyxanthone
Catalog No: CFN92399
CAS No: 6732-85-0
Price: Inquiry(manager@chemfaces.com)
1,3,5,6-Tetrahydroxyxanthone
Catalog No: CFN92539
CAS No: 5084-31-1
Price: Inquiry(manager@chemfaces.com)
1,3,6-Trihydroxy-5-methoxyxanthone
Catalog No: CFN92540
CAS No: 41357-84-0
Price: Inquiry(manager@chemfaces.com)
3,8-Dihydroxy-2,4,6-trimethoxyxanthone
Catalog No: CFN92556
CAS No: 65008-17-5
Price: Inquiry(manager@chemfaces.com)
1,6,7-Trihydroxyxanthone
Catalog No: CFN98284
CAS No: 25577-04-2
Price: Inquiry(manager@chemfaces.com)
Norathyriol
Catalog No: CFN98468
CAS No: 3542-72-1
Price: $368/5mg
Montixanthone
Catalog No: CFN91903
CAS No: 876305-36-1
Price: Inquiry(manager@chemfaces.com)
3,6-Dihydroxy-1,7-dimethoxyxanthone
Catalog No: CFN89303
CAS No: 262292-34-2
Price: Inquiry(manager@chemfaces.com)
Planta Med. 2002 Jan;68(1):49-54.
Natural products inhibiting Candida albicans secreted aspartic proteases from Tovomita krukovii.[Pubmed:
11842327]
METHODS AND RESULTS:
Assay-guided fractionation of the ethanol extract of Tovomita krukovii resulted in the identification of four new xanthones (1 - 4) and ten known compounds (5 - 14). The structures of compounds 1 - 14 were determined by spectral data to be 3,5-dihydroxy-4-methoxyxanthone (1), 1,3,5,7-tetrahydroxy-8-isoprenylxanthone (2), 1,3,5-trihydroxy-8-isoprenylxanthone (3), 1,5,7-trihydroxy-8-isoprenylxanthone (4), 1,3,7-trihydroxy-2-isoprenylxanthone (5), 1,5-dihydroxyxanthone (6), 1,6-dihydroxy-5-methoxyxanthone (7), 1,3,5-trihydroxyxanthone (8), 1,3,6-Trihydroxy-5-methoxyxanthone (9), 1,6-dihydroxy-3,5-dimethoxyxanthone (10), 1,3,7-trihydroxyxanthone (11), 3-geranyl-2,4,6-trihydroxybenzophenone (12), betulinic acid (13), and 3,4-dihydroxybenzoic acid (14).
CONCLUSIONS:
Compounds 2, 3, 12 and 13 showed inhibitory effects against Candida albicans secreted aspartic proteases (SAP) with IC50 values of 15 microg/ml, 25 microg/ml, 40 microg/ml, and 6.5 microg/ml, respectively, while the other compounds were inactive. In addition, compound 12 showed activity against C. albicans, C. neoformans, S. aureus and methicillin resistant S. aureus (MRS).
J Asian Nat Prod Res. 2015;17(4):377-83.
Synthesis and antitumor activity evaluation of a novel series of xanthone derivatives.[Pubmed:
25628155]
METHODS AND RESULTS:
A natural xanthone, 1,3,6-Trihydroxy-5-methoxyxanthone, was totally synthesized for the first time by six steps in 31% overall yield. The xanthone skeleton was formed by a one-step synthesis in 80% yield, and five of its novel derivatives were also obtained by this approach. This synthetic strategy and all the derivatives could be further used for the preparation of other natural xanthones. All the xanthones were characterized by NMR and ESI-MS, and the cytotoxicity of these xanthones was evaluated against HepG2 and HT-29 cells, and the preliminary structure-activity relationship was evaluated from the results.
CONCLUSIONS:
It was proved that the presence of 3-OH group in the molecule is crucial for its biological activity, while the presence of substituents at C-5 and C-6 may also be beneficial.
Nat Prod Res. 2015;29(13):1217-21.
A new flavonoid from Cudrania cochinchinensis.[Pubmed:
25571958 ]
METHODS AND RESULTS:
Chemical investigation of the ethanol extract of the roots of Cudrania cochinchinensis led to the isolation of a new flavonoid, (6S,12S,13R)-1-methoxy cyanomaclurin (1), together with seven known compounds, 1,3,5-trihydroxy-4-(3'-hydroxy-3'-methylbutyl)xanthone (2), 1,3,6-trihydroxy-4-prenylxanthone (3), 1,3,6,7-tetrahydroxyxanthone (4), 1,3,5,6-tetrahydroxyxanthone (5), 1,3,6-Trihydroxy-5-methoxyxanthone (6), resveratrol (7) and oxyresveratrol (8).
CONCLUSIONS:
The structure of compound 1 was elucidated on the basis of 1D and 2D NMR spectra and the HR-ESI-MS data. The absolute stereochemistry was deduced via Rh2(OCOCF3)4-induced CD and NOESY spectra.