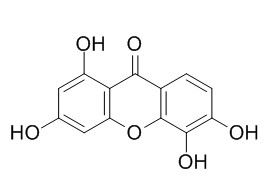
1,3,5,6-Tetrahydroxyxanthone
1,3,5,6-Tetrahydroxyxanthone shows moderate hepatoprotective activity with EC(50) values of 160.2 +/- 0.6 microM against tacrine-induced cytotoxicity in HepG2 cells. It can inhibit angiotensin-I-converting-enzyme activity in a dose-dependent manner.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J. Soc. Cosmet. Sci. Korea2016, 163-171
Int. J. Mol. Sci. 2022, 23(3),1696.
Curr Issues Mol Biol.2024, 46(6):6018-6040.
J Phys Chem Lett.2021, 12(7):1793-1802.
Sci Rep.2024, 14(1):28864.
Phytochem Anal.2021, 32(6):970-981.
Int J Mol Sci.2019, 20(11):E2734
Biomedicine & Pharmacotherapy2020, 125:109950
Exp Parasitol.2015, 153:160-4
Korean Journal of Pharmacognosy.2015, 46(4):352-364
Related and Featured Products
1,5,6-Trihydroxy-3,7-dimethoxyxanthone
Catalog No: CFN89047
CAS No: 65008-02-8
Price: Inquiry(manager@chemfaces.com)
1,5,6-Trihydroxyxanthone
Catalog No: CFN89364
CAS No: 5042-03-5
Price: Inquiry(manager@chemfaces.com)
1,5,6-Trihydroxy-3-methoxyxanthone
Catalog No: CFN89549
CAS No: 50868-52-5
Price: Inquiry(manager@chemfaces.com)
1,3,6-Trihydroxy-2,5-dimethoxyxanthone
Catalog No: CFN96911
CAS No: 345287-92-5
Price: Inquiry(manager@chemfaces.com)
Drimiopsin C
Catalog No: CFN97257
CAS No: 773850-90-1
Price: Inquiry(manager@chemfaces.com)
Drimiopsin D
Catalog No: CFN97258
CAS No: 773850-91-2
Price: Inquiry(manager@chemfaces.com)
Norlichexanthone
Catalog No: CFN98041
CAS No: 20716-98-7
Price: Inquiry(manager@chemfaces.com)
Methyl 1,6-dihydroxy-3-methylxanthone-8-carboxylate
Catalog No: CFN89185
CAS No: 85003-85-6
Price: Inquiry(manager@chemfaces.com)
Eriobofuran
Catalog No: CFN89145
CAS No: 97218-06-9
Price: Inquiry(manager@chemfaces.com)
9-Hydroxyeriobofuran
Catalog No: CFN89153
CAS No: 167278-41-3
Price: Inquiry(manager@chemfaces.com)
Plant Cell Rep. 2015 Jul 21.
Xanthones from roots, hairy roots and cell suspension cultures of selected Hypericum species and their antifungal activity against Candida albicans.[Pubmed:
26194328]
Highest xanthone contents were found in Hypericum pulchrum and H. annulatum untransformed roots. The best anti- Candida activity was obtained for hairy roots extracts of H. tetrapterum clone 2 ATCC 15834.
METHODS AND RESULTS:
Extracts of root cultures, hairy roots and cell suspensions of selected Hypericum spp. were screened for the presence of xanthones and tested for their antifungal activity against Candida albicans strain ATCC 10231. At least one of the following xanthones, 5-methoxy-2-deprenylrheediaxanthone; 1,3,6,7-tetrahydroxyxanthone; 1,3,5,6-Tetrahydroxyxanthone; paxanthone; kielcorin or mangiferin was identified in methanolic extracts of the untransformed root cultures. The highest total xanthone content, with five xanthones, was found in untransformed H. pulchrum and H. annulatum root cultures. Hairy roots and the controls of H. tetrapterum contained 1,7-dihydroxyxanthone, while hairy root cultures and the corresponding controls of H. tomentosum contained toxyloxanthone B, 1,3,6,7- and 1,3,5,6-Tetrahydroxyxanthone. Two xanthones, cadensin G and paxanthone, were identified in cell suspension cultures of H. perforatum. Their content increased about two-fold following elicitation with salicylic acid. The anti-Candida activity of the obtained extracts ranged from MIC 64 to >256 µg ml(-1).
CONCLUSIONS:
Among the extracts of Hypericum untransformed roots, the best antifungal activity was obtained for extracts of H. annulatum grown under CD conditions. Extracts of hairy roots clones A4 and 7 ATCC15834 of H. tomentosum and clone 2 ATCC15834 of H. tetrapterum displayed inhibition of 90% of Candida growth with 256 μg ml(-1). Extracts from chitosan-elicitated cells did not show antifungal activity.
Arch Pharm Res. 2009 Oct;32(10):1393-7
Chromone glycosides and hepatoprotective constituents of Hypericum erectum.[Pubmed:
19898802]
METHODS AND RESULTS:
Two chromone glycosides, hyperimone A [7-(beta-D-glucopyranosyloxy)-5-hydroxy-2-(1-methylethyl)-4H-1-benzopyran-4-one (1)] and hyperimone B [7-(beta-D-glucopyranosyloxy)-5-hydroxy-3-methyl-4H-1-benzopyran-4-one (2)], together with six known compounds were isolated from the methanolic extract of the whole plant of Hypericum erectum.
CONCLUSIONS:
1,3,5,6-Tetrahydroxyxanthone (5) and I3, II8-biapigenin (6) showed moderate hepatoprotective activity with EC(50) values of 160.2 +/- 0.6 microM and 217.7 +/- 1.3 microM, respectively, against tacrine-induced cytotoxicity in HepG2 cells.
J Nat Prod. 1992 May;55(5):691-5.
Inhibition of angiotensin-I-converting enzyme by tetrahydroxyxanthones isolated from Tripterospermum lanceolatum.[Pubmed:
1517742]
METHODS AND RESULTS:
Five tetrahydroxyxanthones, 3,4,6,7-tetrahydroxyxanthone [1], 1,3,5,6-Tetrahydroxyxanthone [2], 3,4,5,6-tetrahydroxyxanthone [3], 1,3,6,7-tetrahydroxyxanthone [4], and 2,3,6,7-tetrahydroxyxanthone [5] isolated from Tripterospermum lanceolatum inhibited angiotensin-I-converting-enzyme activity in a dose-dependent manner.
CONCLUSIONS:
The mode of inhibition of the tetrahydroxyxanthones (THXs) was found to be competitive inhibition. When the tetrahydroxy groups of THXs were blocked with acetyl groups, the angiotensin-I-converting-enzyme inhibitory activity was abolished, suggesting that the tetrahydroxy groups are indispensible for the inhibitory activity.