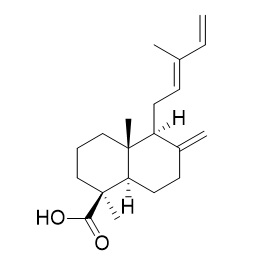
trans-Communic acid
trans-Communic acid exhibits protective effects against UVB-induced skin aging by suppressing UVB-induced MMP-1 expression. trans-Communic acid shows considerable cytotoxicity against four human cancer cell lines in vitro.It also shows good activity against Mycobacterium aurum (MIC of 13.2 microM).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Applied Biological Chemistry2022, 65(77).
Heliyon.2023, e12684.
Am J Chin Med.2015, 30:1-22
Molecules.2023, 28(13):4972.
J Cell Mol Med.2023, jcmm.17968.
J Biomol Struct Dyn.2024, 1-12.
The Catharanthus Genome2022,35-83.
J Breast Cancer.2015, 18(2):112-118
J Nat Prod.2019, 82(4):1002-1008
Environ Toxicol.2024, tox.24246
Related and Featured Products
PLoS One. 2015 Jun 11;10(6):e0128365.
Brown Pine Leaf Extract and Its Active Component Trans-Communic Acid Inhibit UVB-Induced MMP-1 Expression by Targeting PI3K.[Pubmed:
26066652]
Japanese red pine (Pinus densiflora) is widely present in China, Japan, and Korea. Its green pine leaves have traditionally been used as a food as well as a coloring agent. After being shed, pine leaves change their color from green to brown within two years, and although the brown pine leaves are abundantly available, their value has not been closely assessed.
METHODS AND RESULTS:
In this study, we investigated the potential anti-photoaging properties of brown pine leaves for skin. Brown pine leaf extract (BPLE) inhibited UVB-induced matrix metalloproteinase-1 (MMP-1) expression to a greater extent than pine leaf extract (PLE) in human keratinocytes and a human skin equivalent model. HPLC analysis revealed that the quantity of trans-Communic acid (TCA) and dehydroabietic acid (DAA) significantly increases when the pine leaf color changes from green to brown. BPLE and TCA elicited reductions in UVB-induced MMP-1 mRNA expression and activator protein-1 (AP-1) transactivation by reducing DNA binding activity of phospho-c-Jun, c-fos and Fra-1. BPLE and TCA also inhibited UVB-induced Akt phosphorylation, but not mitogen activated protein kinase (MAPK), known regulators of AP-1 transactivation. We additionally found that BPLE and TCA inhibited phosphoinositide 3-kinase (PI3K), the upstream kinase of Akt, in vitro. In summary, both BPLE and its active component TCA exhibit protective effects against UVB-induced skin aging.
CONCLUSIONS:
Taken together, these findings underline the potential for BPLE and TCA to be utilized as anti-wrinkling agents and cosmetic ingredients, as they suppress UVB-induced MMP-1 expression.
J Ethnopharmacol. 2009 Dec 10;126(3):500-5.
Antimycobacterial terpenoids from Juniperus communis L. (Cuppressaceae).[Pubmed:
19755141]
The antimycobacterial activity of Juniperus communis was attributed to a sesquiterpene identified as longifolene (1) and two diterpenes, characterised as totarol (2) and trans-Communic acid (3).
METHODS AND RESULTS:
All compounds were identified following analysis of their spectroscopic data (1D- and 2D-NMR, MS) and by comparison with the literature and commercial authentic standards when available. Revised assignments for 3 are reported. Totarol showed the best activity against Mycobacterium tuberculosis H(37)Rv (MIC of 73.7 microM). It was also most active against the isoniazid-, streptomycin-, and moxifloxacin-resistant variants (MIC of 38.4, 83.4 and 60 microM, respectively). Longifolene and totarol were most active against the rifampicin-resistant variant (MICs of 24 and 20.2 microM, respectively). Totarol showed the best activity in the LORA assay (MIC of 81.3 microM) and against all NTM species (MICs in the range of 7-14 microM). trans-Communic acid showed good activity against Mycobacterium aurum (MIC of 13.2 microM). The low selectivity indices (SI) obtained following cytotoxicity studies indicated that the isolated terpenoids were relatively toxic towards mammalian cells.
CONCLUSIONS:
This is the first report of the isolation of (1) and (2) from Juniperus communis roots, and of (3) from the aerial parts.
The antimycobacterial activity of (1) and (3), and the activity of (2) against Mycobacterium aurum, Mycobacterium fortuitum and Mycobacterium phlei, is reported for the first time. The effect of totarol on drug-resistant variants and non-replicating Mycobacterium tuberculosis has never been published.
Arch Pharm Res. 2011 Dec;34(12):2043-9.
A new lignan glycoside from Juniperus rigida.[Pubmed:
22210029 ]
A new lignan glycoside, named juniperigiside (1) was isolated from the CHCl(3) soluble fraction of the MeOH extract of stems and leaves of Juniperus rigida S.et Z.
METHODS AND RESULTS:
Compound 1 was identified by 1D- and 2D-NMR spectroscopy as well as CD analysis as (2R,3S)-2,3-dihydro-7-methoxy-2-(4'-hydroxy-3'-methoxyphenyl)-3-hydroxymethyl-5-benzofuranpropanol 4'-O-(3-O-methyl)-α-L-rhamnopyranoside. Five known lignans, icariside E4 (2), desoxypodophyllotoxin (3), savinin (4), thujastandin (5), and (-)-nortrachelogenin (6) in addition to five known labdane diterpenes including trans-Communic acid (7), 13-epi-torulosal (8), 13-epi-cupressic acid (9), imbricatoric acid (10), and isocupressic acid (11) were also isolated and their structures were characterized by comparing their spectroscopic data with those in the literature. All compounds were isolated for the first time from this plant, and 5 and 6 were first reported from the genus Juniperus.
The isolated compounds were tested for cytotoxicity against four human tumor cell lines in vitro using a Sulforhodamin B bioassay.
CONCLUSIONS:
Compounds 3, 4, 7, and 8 showed considerable cytotoxicity against four human cancer cell lines in vitro.