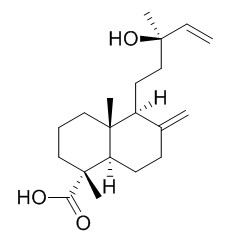
13-Hydroxylabda-8(17),14-dien-18-oic acid
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Journal of Pharmaceutical Investigation2024, 024-00662-1.
Food Sci Biotechnol.2023, 32(9):1215-1223.
Evid Based Complement Alternat Med.2020, 2020:2584783.
J Vet Sci.2020, 21(3):e39.
Biomolecules.2021, 11(10):1537.
Sains Malaysiana2022, 51(4):1143-1154
J Agric Food Chem.2021, 69(46):14037-14047.
J Ethnopharmacol.2020, 260:112988.
Molecules.2021, 26(18):5665.
Exp Biol Med (Maywood).2019, 244(18):1665-1679
Related and Featured Products
Agricultural and Biological Chemistry, 1983, 47(4):787-794.
Microbial Transformation of Manool and (12Z)-Labda- 8(17),12,14-triene by Rhodococcus erythropolis JTS-131.[Reference:
WebLink]
Rhodococcus erythropolis JTS-131, a cis-abienol and sclareol transformable bacterium, converted manool and accumulated manoyl oxide in the culture broth.
METHODS AND RESULTS:
In the presence of metabolic inhibitors, 13β-hydroxylabda-8(17),14-dien-18-oic acid(13-Hydroxylabda-8(17),14-dien-18-oic acid) and its methylester (a new compound) were accumulated as the transformation products. This strain also transformed (12Z)-labda-8(17),12,14- triene and acqumulated (12Z)-labda-8(17),12,14-trien-18-al in the culture broth. In the presence of metabolic inhibitors, two compounds, (12Z)-labda-8(17),12,14-trien-18-ol and 4,18,19-trinor-3,4- seco-5-oxo-(12Z)-labda-8(17),12,14-trien-3-oic acid were accumulated.
CONCLUSIONS:
Based on the experiments on the degradation sequence, transformation pathways for these two labdanes by the bacterium are proposed.