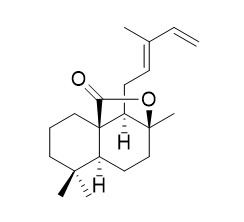
12E,14-Labdadien-20,8beta-olide
12E,14-Labdadien-20,8beta-olide is a natural product from Isodon yuennanensis.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Enzyme Microb Technol.2022, 153:109941.
Food Analytical Methods2020, 1-10
Yakugaku Zasshi.2018, 138(4):571-579
Planta Med.2023, a-2192-2281.
PLoS One.2020, 15(2):e0220084.
Microb Biotechnol.2021, 14(5):2009-2024.
Food Bioscience2024, 58:103691.
bioRxiv2021, 458409.
Nutrients.2019, 11(11):E2694
Biol Pharm Bull.2023, 46(2):245-256.
Related and Featured Products
Angew Chem Int Ed Engl. 2017 May 15;56(21):5844-5848.
Bioinspired Asymmetric Synthesis of Hispidanin A.[Pubmed:
28332749 ]
METHODS AND RESULTS:
The first enantiospecific synthesis of hispidanin A (4), a dimeric diterpenoid from the rhizomes of Isodon hispida, was achieved with a longest linear sequence of 12 steps in 6.5 % overall yield. A key component is the use of the abundant and naturally occurring diterpenoids (+)-sclareolide and (+)-sclareol as starting materials, which enables the gram-scale preparation of the key intermediates totarane (1) and s-trans-12E,14-Labdadien-20,8beta-olide (2). Subsequently a thermal or an erbium-catalyzed intermolecular Diels-Alder reaction of totarane (1) with labdadienolide (2) provide convergent and rapid access to the natural product hispidanin A (4).
CONCLUSIONS:
The synthetic studies have offered significant impetus for the efficient construction of these architecturally complex natural products.
Nat Prod Res. 2015;29(7):628-32.
Two new labdane diterpenoids from the rhizomes of Isodon yuennanensis.[Pubmed:
25420949 ]
METHODS AND RESULTS:
Two new labdane diterpenoids, s-trans-8(17),12E,14-labdatrien-20-oic acid (1), s-trans-12E,14-Labdadien-20,8beta-olide (2), along with 10 known compounds, hinokiol (3), ursonic acid (4), 2α,3α-dihydroxyolean-12-en-28-oic acid (5), 2α,3β,23-trihydroxyolean-12-en-28-oic acid (6), ethyl 3-(3,4-dihydroxyphenyl)lactate (7), ethyl rosmarinate (8), (Z,E)-2-(3,4-dihydroxyphenyl)ethenyl caffeic ester (9), tridecanoic acid (10), β-sitosterol (11) and daucosterol (12), were isolated from the 70% acetone extract of the rhizomes of Isodon yuennanensis.
CONCLUSIONS:
Their structures were elucidated based on the analyses of extensive spectroscopic data and physicochemical properties.