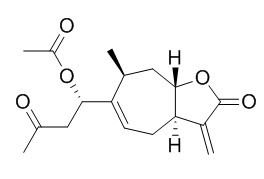
Xanthinin
Xanthinin is a plant growth-regulating compound from Xanthium pennsylvanicum, it is also a phytotoxic xanthanolide, can inhibit the growth of plants.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biomed Pharmacother.2021, 139:111585.
Planta Medica International2022, 9(01):e108-e115.
J Microbiol Immunol Infect.2021, S1684-1182(21)00142-0.
Int J Mol Sci.2023, 24(8):7045.
Agriculture2024, 14(12), 2301
J.Food Pharm.Sci.2024, 12(2), 116-124.
Plant Cell Physiol.2018, 59(1):128-141
Plants2022, 11(3),294.
Chem Biol Interact.2024, 398:111103.
Primary and Industrial.2018, 52(11)
Related and Featured Products
Molecules. 2015 Apr 17;20(4):7034-47.
Phytochemical compositions and biological activities of essential oil from Xanthium strumarium L.[Pubmed:
25898416]
The chemical composition of the essential oil (EO) from fresh cocklebur (Xanthium strumarium L.) leaves was investigated by GC-MS.
METHODS AND RESULTS:
The antimicrobial activity of the EO was tested against Gram-positive and Gram-negative bacteria and fungi. Scolicidal activity was assayed against Echinococcus granulosus protoscolices. In total, 34 compounds were identified, accounting for 98.96% of the EO. The main compounds in the EO were cis-β-guaiene (34.2%), limonene (20.3%), borneol (11.6%), bornyl acetate (4.5%), β-cubebene (3.8%), sabinene (3.6%), phytol (3.1%), β-selinene (2.8%), camphene (2.2%), α-cubebene (2.4%), β-caryophyllene (1.9%), α-pinene (1.8%) and Xanthinin (1.04%). The antibacterial and antifungal screening of the EO showed that all assayed concentrations significantly inhibited the growth of Staphylococcus aureus, Bacillus subtilis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Candida albicans and Aspergillus niger (MIC = 0.5 ± 0.1, 1.3 ± 0.0, 4.8 ± 0.0, 20.5 ± 0.3, 55.2 ± 0.0 and 34.3 ± 0.0 µg/mL, respectively). The scolicidal assay indicated that the EO exhibited a significant activity against E. granulosus protoscolices.
CONCLUSIONS:
To the best of our knowledge, this is the first report on the scolicidal activity of X. strumarium. Because of the emergence of antimicrobial drug resistance, the study of new effective natural chemotherapeutic agents, such as the X. strumarium EO, possibly with low side effects, represents a very promising approach in biomedical research.
Eur J Med Chem. 2015 Jan 27;90:491-6.
Optimization of xanthatin extraction from Xanthium spinosum L. and its cytotoxic, anti-angiogenesis and antiviral properties.[Pubmed:
25481815]
METHODS AND RESULTS:
The aqueous extraction of the sesquiterpene lactone xanthatin from Xanthium spinosum L. favours the conversion of Xanthinin (1) to xanthatin (2) via the loss of acetic acid. The cytotoxic (Hep-G2 and L1210 human cell lines) and antiviral activities of isolated xanthatin are established.
CONCLUSIONS:
This natural compound shows significant cytotoxicity against the Hep-G2 cell line and our experimental results reveal its strong anti-angiogenesis capacity in vitro. The structure of xanthatin is determined by spectroscopic methods and for the first time confirmed by X-ray diffraction.
Allelopathy J., 2015, 35(1):77-86.
Selective phytotoxicity of xanthinin and xanthatin from invasive weed Xanthium italicum Morretti on test plants[Reference:
WebLink]
METHODS AND RESULTS:
Two phytotoxic xanthanolides, Xanthinin and xanthatin, were isolated from the leaves and fruits of invasive weed, Xanthium italicum Morretti, commonly known as Italian cocklebur. They slightly inhibited the seedling growth of Italian cocklebur, but were very inhibitory to lettuce, ryegrass and two indigenous species (African rue and redroot pigweed). Xanthinin significantly decreased the growth of lettuce (Lectuca sativa L.), ryegrass (Lolium multiforum Lam.), Syrian me (Peganum harmala L.) and redroot pigweed (Amaranthus retroflexus L.) even at low concentration (10 mu g/mL). In contrast, the root length of Italian cocklebur slightly inhibited (2%) compared to control at 50 mu g/mL Xanthinin treatment. The application of xanthatin also inhibited the growth of receiver plants. Ryegrass and Syrian rue were most sensitive species, whose root growth was inhibited by 78% and 96%, respectively, at 50 mu g/mL dose of xanthatin, while the root length of Italian cocklebur was only inhibited by 23%. In fact, Italian cocklebur was the only receiver species that survived at 1 mg/mL xanthatin and Xanthinin treatment.
CONCLUSIONS:
Our results suggested that the selective phytotoxicity of Italian cocklebur and other species facilitated its successful invasion. This is the first report on the phytotoxic activity of xanthatin.
Tree Physiol., 2008, 28(5):665-78.
Xanthinin: a Plant Growth-regulating Compound from Xanthium pennsylvanicum.[Reference:
WebLink]
METHODS AND RESULTS:
A crystalline compound, Xanthinin, has been isolated from the leaves of Xanthium pennsylvanicum (Compositae). Xanthinin is an unsaturated keto lactone with the composition C17H22O5, and can be converted by the loss of a molecule of acetic acid into the previously reported xanthatin, C15H18O3. Xanthinin and xanthatin appear to be related to the lactones already known in other Compositae: helenalin, tenulin and the santonins.