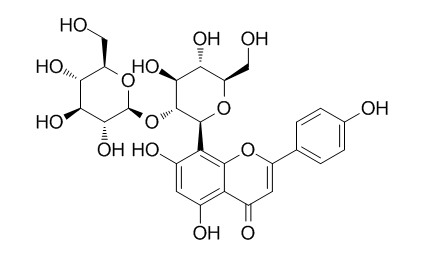
Vitexin 2''-glucoside
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Chem.2024, 458:140201.
Sustainable Chemistry & Pharmacy2022, 30:100883.
Sci Rep.2023, 13(1):13072.
Antioxidants (Basel).2023, 13(1):12.
Vietnam Journal of Food Control2022, 5(3):pp.390-401.
Food Bioscience2024, 58:103691.
The Catharanthus Genome2022,35-83.
Int Immunopharmacol.2021, 100:108073.
Proc. Sci. Math.2024, V25:108-123
Toxicol Rep.2021, 8:1131-1142.
Related and Featured Products
Journal of separation science, 2015, 30(5):717-721.
Simultaneous determination of vitexin-2[Reference:
WebLink]
METHODS AND RESULTS:
RP-HPLC with UV photodiode array detection (UV-DAD) was developed and validated for the simultaneous determination of vitexin-2"-O-glucoside(Vitexin 2''-glucoside), vitexin-2"-O-rhamnoside, rutin, and hyperoside in the extract of hawthorn (Crataegus pinnatifida Bge.) leaves. The analytes of interest were separated on a Diamonsil C18 column (250 x 4.6 mm id, 5 microm) with the mobile phase consisting of THF/ACN/methanol/ 0.05% phosphoric acid solution (pH 5.0) (18:1:1:80 v/vl/v). The flow rate was set at 1.0 mL/min and the eluent was detected at 340 nm for the four flavonoids. The method was linear over the studied range of 1.00-100 microg/mL for the four analytes of interest with the correlation coefficient for each analyte greater than 0.999. The LOD and LOQwere 0.03 and 0.10 microg/mL, 0.03 and 0.10 microg/mL, 0.05 and 0.15 pg/mL, 0.10 and 0.30 microg/mL for vitexin-2"-O-glucoside, vitexin-2"-0-rhamnoside, rutin, and hyperoside, respectively.
CONCLUSIONS:
The optimized method was successfully applied to the analysis of four important flavonoids in the extract of hawthorn leaves. The total amounts of the four flavonoids were 22.2, 62.3, 4.27, and 8.24 mg/g dry weight for vitexin-2"-O-glucoside, vitexin-2"-O-rhamnoside, rutin, and hyperoside in the extract of hawthorn leaves, respectively.