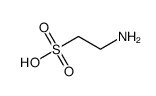
Taurine
Taurine, a free β-amino acid with remarkable antioxidant activity, is used in Taurine-enriched beverages to boost the muscular power of athletes. Taurine can effectively promote chondrocyte growth and enhance accumulation of glycosaminoglycans and collagens in the conditioned media of chondrocytes, it is effective in proliferation promotion and phenotype maintenance of chondrocytes, thus, taurine may be a useful pro-chondrogenic agent for autologous chondrocyte implantation in the treatment of cartilage repair.Taurine also can attenuate nandrolone decanoate-induced poor sperm quality and testicular toxicity in rats.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Exp Biol Med (Maywood).2019, 244(16):1463-1474
Am J Chin Med.2015, 30:1-22
Molecules.2021, 26(4):817.
Environ Toxicol.2024, 39(4):2417-2428.
Antioxidants (Basel).2022, 11(8):1471.
Antioxidants (Basel).2022, 11(12):2496.
Toxins (Basel).2022, 14(12):824.
Biomol Ther (Seoul).2020, 28(6):542-548.
Food Chem.2018, 252:207-214
Trop J Nat Prod Res2023, 7(12):5611-5615.
Related and Featured Products
Tohoku J Exp Med. 2015;235(3):201-13.
Chondroprotective effects of taurine in primary cultures of human articular chondrocytes.[Pubmed:
25765089]
METHODS AND RESULTS:
We explored the effect of Taurine (2-aminoethane sulfonic acid) on proliferation and phenotype maintenance of human articular chondrocytes by analyzing the cell proliferation, morphology, viability, and expression of cartilage specific mRNAs and proteins. Primary chondrocytes were isolated from human articular cartilage tissues. Results showed that Taurine effectively promoted chondrocyte growth and enhanced accumulation of glycosaminoglycans and collagens in the conditioned media of chondrocytes. Moreover, Taurine exposure caused significant increases in the relative expression levels of mRNAs for cartilage specific markers, including aggrecan, collagen type II and SOX9. Aggrecan is a cartilage-specific proteoglycan, and SOX9 is a chondrogenic transcription factor. In contrast, the mRNA expression of collagen type I, a marker for chondrocyte dedifferentiation, was significantly decreased in cells treated with Taurine, indicating that Taurine inhibits the chondrocyte dedifferentiation.
CONCLUSIONS:
This study reveals that Taurine is effective in proliferation promotion and phenotype maintenance of chondrocytes. Thus, Taurine may be a useful pro-chondrogenic agent for autologous chondrocyte implantation in the treatment of cartilage repair.
Toxicol Appl Pharmacol. 2015 Feb 1;282(3):285-96.
Amelioration of nandrolone decanoate-induced testicular and sperm toxicity in rats by taurine: effects on steroidogenesis, redox and inflammatory cascades, and intrinsic apoptotic pathway.[Pubmed:
25542992]
Taurine; a free β-amino acid with remarkable antioxidant activity, is used in Taurine-enriched beverages to boost the muscular power of athletes. Therefore, the purpose of this study was to investigate the mechanisms of the possible protective effects of Taurine on nandrolone decanoate-induced testicular and sperm toxicity in rats.
METHODS AND RESULTS:
To achieve this aim, male Wistar rats were randomly distributed into four groups and administered either vehicle, nandrolone decanoate (10mg/kg/week, I.M.), Taurine (100mg/kg/day, p.o.) or combination of Taurine and nandrolone decanoate, for 8 successive weeks. Results of the present study showed that Taurine reversed nandrolone decanoate-induced perturbations in sperm characteristics, normalized serum testosterone level, and restored the activities of the key steroidogenic enzymes; 3β-HSD, and 17β-HSD. Moreover, Taurine prevented nandrolone decanoate-induced testicular toxicity and DNA damage by virtue of its antioxidant, anti-inflammatory, and anti-apoptotic effects. This was evidenced by Taurine-induced modulation of testicular LDH-x activity, redox markers (MDA, NO, GSH contents, and SOD activity), inflammatory indices (TNF-α, ICAM-1 levels, and MMP-9 gene expression), intrinsic apoptotic pathway (cytochrome c gene expression and caspase-3 content), and oxidative DNA damage markers (8-OHdG level and comet assay).
CONCLUSIONS:
In conclusion, at the biochemical and histological levels, Taurine attenuated nandrolone decanoate-induced poor sperm quality and testicular toxicity in rats.
Am J Physiol Gastrointest Liver Physiol. 2015 Feb 15;308(4):G277-86.
Estradiol decreases taurine level by reducing cysteine sulfinic acid decarboxylase via the estrogen receptor-α in female mice liver.[Pubmed:
25394658]
Cysteine sulfinic acid decarboxylase (CSAD) and cysteine dioxygenase (CDO) are two rate-limiting enzymes in Taurine de novo synthesis, and their expressions are associated with estrogen concentration.
METHODS AND RESULTS:
The present study was designed to determine the relationship between 17β-estradiol (E₂) and Taurine in female mice liver. We initially observed the mice had lower levels of CSAD, CDO, and Taurine during estrus than diestrus. We then, respectively, treated the ovariectomized mice, the cultured hepatocytes, and Hep G2 cells with different doses of E₂, and the CSAD and CDO expressions and Taurine levels were analyzed. The results showed that E₂ decreased Taurine level in the serum and the cultured cells by inhibiting CSAD and CDO expressions. Furthermore, we identified the molecular receptor types through which E₂ plays its role in regulating Taurine synthesis, and our results showed that estrogen receptor-α (ERα) expression was much higher than estrogen receptor-β (ERβ) in the liver and hepatocytes, and the inhibiting effects of E₂ on CSAD, CDO, and Taurine level were partially abrogated in the ICI-182,780-pretreated liver and hepatocytes, and in ERα knockout mice.
CONCLUSIONS:
These results indicate that estradiol decreases Taurine content by reducing Taurine biosynthetic enzyme expression in mice liver.
Am J Physiol Heart Circ Physiol. 2015 Feb 1;308(3):H232-9.
Role of protein phosphorylation in excitation-contraction coupling in taurine deficient hearts.[Pubmed:
25437920]
Taurine is a beta-amino acid found in very high concentration in the heart. Depletion of these intracellular stores results in the development of cardiomyopathy, thought to be mediated by abnormal sarcoplasmic reticular (SR) Ca(2+) transport.
METHODS AND RESULTS:
There is also evidence that Taurine directly alters the Ca(2+) sensitivity of myofibrillar proteins. Major regulators of SR Ca(2+) ATPase (SERCA2a) are the phosphorylation status of a regulatory protein, phospholamban, and SERCA2a expression, which are diminished in the failing heart. The failing heart also exhibits reductions in myofibrillar Ca(2+) sensitivity, a property regulated by the phosphorylation of the muscle protein, troponin I. Therefore, we tested the hypothesis that Taurine deficiency leads to alterations in SR Ca(2+) ATPase activity related to reduced phospholamban phosphorylation and expression of SERCA2a. We found that a sequence of events, which included elevated protein phosphatase 1 activity, reduced autophosphorylation of CaMKII, and reduced phospholamban phosphorylation, supports the reduction in SR Ca(2+) ATPase activity. However, the reduction in SR Ca(2+) ATPase activity was not caused by reduced SERCA2a expression. Taurine transporter knockout (TauTKO) hearts also exhibited a rightward shift in the Ca(2+) dependence of the myofibrillar Ca(2+) ATPase, a property that is associated with an elevation in phosphorylated troponin I.
CONCLUSIONS:
The findings support the observation that Taurine deficient hearts develop systolic and diastolic defects related to reduced SR Ca(2+) ATPase activity, a change mediated in part by reduced phospholamban phosphorylation.
Exp Toxicol Pathol. 2015 Jan;67(1):13-20.
The effects of taurine on vigabatrin, high light intensity and mydriasis induced retinal toxicity in the pigmented rat.[Pubmed:
25446799]
The overall purpose of this study was to establish a model that may be used for examining the effect of Vigabatrin-induced retinal toxicity in pigmented rats, and subsequently examine the possible effects of Taurine on the retinal toxicity.
METHODS AND RESULTS:
In the first part of the study, pigmented Long Evans rats were subjected to combinations of induced mydriasis, low/high light intensities (40/2000 lx) and oral administration of near-MTD (Maximum Tolerated Dose) doses (200 mg/kg/day) of Vigabatrin for up to 6 weeks. The combination of mydriasis and high light intensity applied to Long Evans rats resulted in retinal damage that was increased by the administration of Vigabatrin. In the second part of the study Long Evans rats were subjected to combinations of induced mydriasis and high/low light intensity (40/2000 lx) while being orally administered low (30 mg/kg/day) or high (200 mg/kg/day) doses of Vigabatrin for up to 6 weeks. In addition, selected groups of animals were administered Taurine via the drinking water (20 mg/ml), resulting in systemic Taurine concentrations of approximately threefold the endogenous concentration. The combined results of the studies demonstrate that retinal damage can be induced in pigmented animals when combining mydriasis and high light intensity. Retinal damage was functionally evaluated by electroretinography (ERG), then confirmed by histopathology. While depending on mydriasis and high light intensity, administration of Vigabatrin increased the retinal toxicity and resulted in the formation of rosette-like structures in the retina in a dose-related manner.
CONCLUSIONS:
Administration of Taurine did not alleviate the Vigabatrin-induced retinal toxicity, as demonstrated either functionally by ERG or morphologically, although systemic concentrations of 3-fold the endogenous levels were reached, and it was thus not possible to demonstrate a protective effect of Taurine in these pigmented animals.