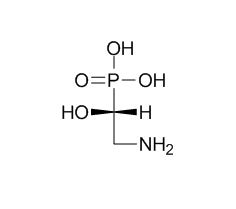
(2-Amino-1-hydroxyethyl)phosphonic acid
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Tumour Biol.2015, 36(12):9385-93
Food and Agriculture Org. Of the UN2019, 151-160
J Herbmed Pharmacol.2018, 7(4):280-286
Plant Archives2020, 2(1),2929-2934
Biomedicines.2024, 12(3):495.
Nutrients.2018, 10(10)
J Korean Med Ophthalmol Otolaryngol Dermatol2023, 36(1):21-39.
JAOCS2021, 98(7):779-794.
Nat Chem Biol.2018, 14(8):760-763
Food Funct.2022, 13(14):7638-7649.
Related and Featured Products
Acs Catalysis, 2017:3521-3531.
Mechanism of Organophosphonate Catabolism by Diiron Oxygenase PhnZ: A Third Iron-Mediated O-O Activation Scenario in Nature.[Reference:
WebLink]
Diiron oxygenase PhnZ catalyzes the catabolism of organophosphonate (Pn) (R)-2-amino-1-hydroxyethylphosphonic((2-Amino-1-hydroxyethyl)phosphonic acid) to glycine and inorganic phosphate (Pi).
METHODS AND RESULTS:
In this Pn catabolism way, PhnZ oxidatively cleaves the highly stable C-P bond in Pn to produce Pi. However, the mechanism of this enzyme that affords aquatic and marine bacteria in Pi-limited environments to utilize the most abundant environmental Pn (2-amino-ethylphosphonic acid) as the source of Pi, is still unclear. In this work, extensive QM/MM calculations reveal that the mechanism of PhnZ consists of four consecutive steps: (1) Rate-limiting α-H abstraction of Pn by FeIII-superoxo; (2) Formation of FeIIIOOCα peroxide; (3) Concerted O insertion into Cα-P bond of Pn initiated by “inverse” heterolytic O-O cleavage; (4) Phosphate hydrolysis to glycine and Pi. Intriguingly, the enzymatic reaction mechanism of PhnZ for the crucial breakage of the C-P bond is characterized by the “inverse” heterolytic O-O cleavage of FeIIIOOCα intermediate, which renders the distal O atom more oxidative to oxygenate Pn than the homolytic O-O cleavage.
CONCLUSIONS:
In this way, PhnZ adopts a mechanism quite different from the related diiron oxygenase MIOX, with His62 residue playing an important role. This unusual “inverse” heterolytic O-O cleavage mode, apart from the well-known homolytic and “normal” heterolytic ones, constitutes a third iron-mediated O-O activation scenario in nature, which is expected to have its broad occurrence in oxidative transformation involving heteroatoms of sulfur and phosphorus.