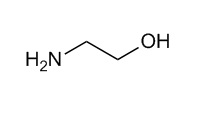
2-Aminoethanol
2-Aminoethanol (ethanolamine) can inhibit the GABA degrading enzyme GABA aminotransferase (GABA-T), it is a possible neuromodulator in the pigeon optic tectum. Strengthening of the antioxidant mechanisms by plant pretreatment with 2-aminoethanol could be helpful in the development of a broad tolerance to adverse stress conditions.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Key Engineering Materials2022, 931(47-53).
Nutrients.2018, 10(12):E1998
BMC Complement Altern Med.2018, 18(1):303
Molecules. 2013, 18(11):14105-21
Pest Manag Sci.2019, 75(9):2530-2541
Chinese Medicine2019, 14(1)
Plants (Basel).2023, 12(22):3877.
Research Square2021, 10.21203.
Food Hydrocolloids2024, 152:109898
Oncotarget.2017, 8(53):90925-90947
Related and Featured Products
Russian Journal of Plant Physiology, 2011,58 (1):45-50.
Effect of 2-aminoethanol pretreatment on the antioxidant enzyme activity in Zea mays under oxidative stress[Reference:
WebLink]
Exposure of plants to stress may result in liberation of 2-Aminoethanol as an inducer of alarm reaction that activates cellular resistance and tolerance mechanisms.
METHODS AND RESULTS:
In the present study, the effect of exogenous 2-Aminoethanol on stimulation of tolerance mechanisms was investigated in maize (Zea mays L.) plants treated with the herbicide paraquat. Maize shoots were pretreated with 2-Aminoethanol. Two days later the shoots were treated with paraquat. Pretreatment with 2-Aminoethanol increased catalase and guaiacol peroxidase activities and cysteine content and decreased MDA and hydrogen peroxide content in maize plants treated with paraquat.
CONCLUSIONS:
Based on the results of this study, it can be suggested that strengthening of the antioxidant mechanisms by plant pretreatment with amino alcohol could be helpful in the development of a broad tolerance to adverse stress conditions.
Neurosci Lett. 1982 Sep 20;32(1):53-8.
2-Aminoethanol as a possible neuromodulator in the pigeon optic tectum.[Pubmed:
7145226]
METHODS AND RESULTS:
A mass fragmentographic method was used for determination of low molecular weight compounds in perfusates collected in vivo in the pigeon optic tectum by a push-pull cannula technique. 2-Aminoethanol (ethanolamine) could be collected under resting conditions (5.6 +/- 0.09 pmol/min). Electrical stimulation of optic nerve induced a 2.3-fold increase of the tectal ethanolamine outflow whereas that of GABA was not affected. Ethanolamine applied iontophoretically to tectal neurons did not influence their spontaneous discharge; however, their glutamate-induced excitation as well as the GABA-induced depression were enhanced if ethanolamine was applied simultaneously.
CONCLUSIONS:
It is suggested that optic nerve stimulation exerts a neuromodulatory effect on tectal neurons.
Neurosci Lett. 1983 Dec 11;42(3):293-7.
Effect of 2-aminoethanol on the synthesis, binding, uptake and metabolism of GABA[Pubmed:
6320073]
2-Aminoethanol (ethanolamine) was studied for effects on neurochemical assays for GABA synthesis, receptor binding, uptake and metabolism in rat brain preparations.
METHODS AND RESULTS:
The effects of ethanolamine were compared with those of ethanolamine O-sulphate (EOS), an inhibitor of GABA degradation. Furthermore, the effect of both compounds was compared on GABA metabolism in rat brain in vivo. In vitro, ethanolamine and EOS had no significant effect on the GABA synthesizing enzyme glutamic decarboxylase (GAD) and GABA uptake, but both drugs proved virtually equipotent to inhibit the GABA degrading enzyme GABA aminotransferase (GABA-T). EOS was a relatively potent inhibitor of GABA receptor binding, whereas ethanolamine was not effective in this regard. Following systemic administration in rats, 50% inhibition of GABA-T in the brain was achieved by 500 mg/kg ethanolamine or 2000 mg/kg EOS, respectively. As a consequence of GABA-T inhibition, GABA levels increased significantly. GAD activity remained unchanged after both treatments.
CONCLUSIONS:
The present results suggest that the recently reported enhancement of functional effects of GABA by ethanolamine may relate, at least in part, to the inhibitory effect of the compound on GABA catabolism.
J. Phys. Chem. A, 2002, 106 (4), pp 668–679
Hydrogen Bonding in Monomers and Dimers of 2-Aminoethanol[Reference:
WebLink]
METHODS AND RESULTS:
Equilibrium structures of monomers and dimers of 2-Aminoethanol (AE) exhibiting different intramolecular and intermolecular hydrogen bonds between the OH and NH2 groups were optimized and analyzed in theoretical density functional B3LYP/6-311++G(2d,2p) calculations. Natural bond orbital (NBO) theory was applied to quantify the relative strength of these interactions and to account for their effect on stability, structural, and vibrational parameters of both monomers and dimers. It is shown that the charge transferred from the lone pair of the hydrogen bond acceptor to the antibonding orbital of the donor provides the substantial stabilizing component of the hydrogen bond. NBO energetic analysis demonstrates that the OH···N interaction is the strongest one for both monomers (intramolecular) and dimers (intermolecular). The intramolecular hydrogen bond in AE monomers is relatively weak, in part, because of its bent nature. The formation of a stronger and more linear intermolecular hydrogen bond between molecular units in AE dimers is accompanied by cooperative enhancement of the intramolecular hydrogen-bonding interactions.
CONCLUSIONS:
This effect is explained in terms of charge transfer among local bond orbitals and is relevant to the cooperative strengthening of hydrogen-bonding interactions in larger AE clusters.