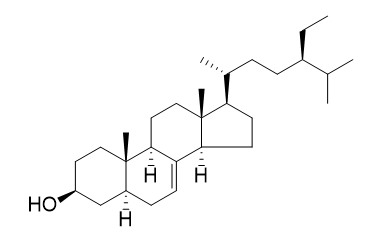
Stigmast-7-en-3-ol
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Phys Chem Lett.2021, 12(7):1793-1802.
Journal of Food Composition and Analysis2021, 100:103905.
Materials Today Communications2023, 37:107216
Agriculture2024, 14(12), 2301
BMC Plant Biol.2022, 22(1):128.
Carbohydrate Polymer Technologies & App.2021, 2:100049.
Mol Pharmacol.2021, 99(2):163-174.
BMC Complement Altern Med.2019, 19(1):367
Phytomedicine.2019, 58:152893
Prev Nutr Food Sci.2024, 29(4):563-571.
Related and Featured Products
J Sep Sci. 2014 Jun;37(11):1308-14.
Extraction optimization by response surface methodology: Purification and characterization of phytosterol from sugarcane (Saccharum officinarum L.) rind.[Pubmed:
24648272 ]
A green, simple, and effective method for the extraction of sugarcane lipids from sugarcane rind was investigated by response surface methodology.
METHODS AND RESULTS:
The optimum conditions of technological progress obtained through response surface methodology were as follows: liquid-to-solid ratio 7.94: 1 mL/g, extraction temperature 50°C and extraction time 5.98 h. The practical sugarcane lipids extraction yield was 6.55 ± 0.28%, which was in good consistence with the predicted extraction yield of 6.47%. The results showed that the sugarcane lipids extraction yield obtained in optimum conditions increased by 1.16∼7.28-fold compared to the yields obtained in single-factor experiments. After saponification and SPE steps, the nonsaponifiable fraction of sugarcane lipids was analyzed by gas chromatography with mass spectrometry and high-performance liquid chromatography. β-Sitosterol, stigmasterol, and campesterol were the prevailing phytosterols in the sample, while fucosterol, gramisterol, Stigmast-7-en-3-ol, (3β,5α,24S)-, stigmasta-4,6,22-trien-3α-ol, and cholest-8(14)-en-3β-ol acetate were also identified as minor steroids. Furthermore, the content of β-sitosterol and a mixture of campesterol and stigmasterol (quantified by high-performance liquid chromatography) was 44.18 mg/100 g dry weight and 43.20 mg stigmasterol/100 g dry weight, respectively.
CONCLUSIONS:
Our results indicate that sugarcane rind is a good source of phytosterol.