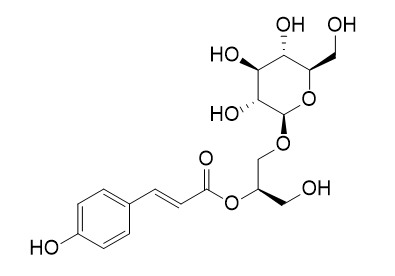
Regaloside H
Regaloside H, a phenylpropanoid glycerol glucoside, is a gluconeogenesis inhibitor. Regaloside H can reduce glucose production in Hepatocytes
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J of the Korean Society of Cosmetics and Cosmetology2019, 225-231
Analytical Methods2018, 10(27)
Antioxidants (Basel).2020, 9(2):E120
SSRN2024, 4937625.
J Integr Plant Biol.2023, 13564.
Revista Brasileira de Farmacognosia2024, 34:1276-1286.
Food Chem.2024, 452:139555.
Phytomedicine.2023, 117:154929.
J Cell Mol Med.2022, 26(23):5807-5819.
Heliyon.2023, e12778.
Related and Featured Products
ACS Omega . 2019 Jun 19;4(6):10670-10676.
Phenylpropanoid Glycerol Glucosides Attenuate Glucose Production in Hepatocytes[Pubmed:
31460164]
An activity-guided fractionation approach revealed several phenylpropanoid glycerol glucosides isolated from the bulbs of Lilium longiflorum Thunb. (Easter lily) with gluconeogenesis inhibitory activities. The strongest activity was observed for (2S)-1-O-p-coumaroyl-2-O-β-d-glucopyranosylglycerol (3), (2S)-1-O-caffeoyl-2-O-β-d-glucopyranosylglycerol (1), and (2R)-1-O-β-d-glucopyranosyl-2-O-p-coumaroylglycerol (2) with inhibitions of 51.2, 39.2, and 36.8%, respectively. The p-coumaroyl-based (3) and its acetylated derivative (5) exhibited differential inhibition activity (51.2% as compared to 3.6%), suggesting that natural acetylation decreases the hypoglycemic activity of these compounds. Direct structure-activity analysis of phenylpropanoid glycerol glucosides indicated that the hydroxylation pattern of the hydroxy cinnamic acid moiety and acetylation were responsible for the differences in activity. This is the first report of phenylpropanoid glycerol glucosides as a phytochemical class of hepatic glucose production inhibitors.