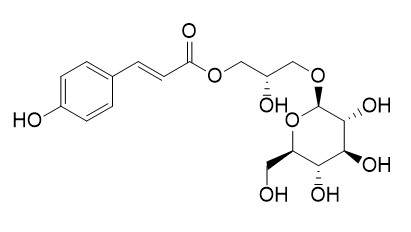
Regaloside A
Regaloside A has anti-inflammatory activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Foods. 2022, 11(23):3905.
Processes2020, 8(12),1540.
Environ Toxicol.2020, doi: 10.1002
J Med Food.2019, 22(10):1067-1077
Evid Based Complement Alternat Med.2021, 2021:6687513.
Life (Basel).2023, 13(2):457.
Aquaculture2017, 481:94-102
Pharmaceutics.2022, 14(5):945.
TCI CO.2019, US20190151257A1
Res Pharm Sci.2023, 18(3):244-261.
Related and Featured Products
Applied Biological Chemistry volume 60, pages527–533 (2017)
Phenylpropanoids from Lilium Asiatic hybrid flowers and their anti-inflammatory activities[Reference:
WebLink]
Three phenylpropanoids were isolated from the flowers of Lilium Asiatic hybrids through repeated silica gel or octadecyl silica gel column chromatographies. The chemical structures were determined to be 1-O-trans-caffeoyl-β-D-glucopyranoside (1), Regaloside A (2), and regaloside B (3), based on spectroscopic data gathered from nuclear magnetic resonance (NMR) spectroscopy, electron ionization mass spectrometry (EI/MS), polarimetry, and infrared spectroscopy (IR) experiments. Compounds 1 and 2 showed significant DPPH radical scavenging activity of 60.1 and 58.0% at 160 ppm, respectively, compared with the 62.0% activity of the positive control, α-tocopherol. At a concentration of 50 μg/mL, compounds 1–3 inhibited the expression of iNOS to 4.1 ± 0.01, 70.3 ± 4.07, and 26.2 ± 0.63, respectively, and decreasing COX-2 expression to 67.8 ± 4.86, 131.6 ± 8.19, and 98.9 ± 4.99. Also, at the same concentration, compounds 1–3 decreased the ratio of p-p65/p-65 to 43.8 ± 1.67, 40.7 ± 1.30, and 43.2 ± 1.60, respectively, and the expression of VCAM-1 to 42.1 ± 2.31, 48.6 ± 2.65, and 33.8 ± 1.74, respectively.
China Journal of Chinese Materia Medica, 2007, 32(16):1656-9.
Studies on chemical constituents in fresh fleshy scaleleaf of Lilium lancifolium.[Pubmed:
18027661]
To study the chemical constituents in fresh fleshyscaleaf of Lilium lancifolium.
METHODS AND RESULTS:
The constituents were separated. by various kinds of chromatography and their structures were identified on the basis of spectral analysis. Ten compounds were identified Regaloside A (1), regaloside C (2), methyl-a-D-mannopyranosid (3), methyl-ca-D-glucopyranoside (4), (25R, 26R) -26-methoxyspirost-5-ene-3p-yl-O-ca-L-rhamnopyranosyl-(1-->2)-[beta-D-glucopyranosyl-(1-->6)]-beta-D-glucopyranoside (5), (25R)-spirost-5-ene-3beta-yl-O-alpha-L-rhamnopyranosyl-(1-->2)-[beta-D-glucopyranosyl-(1-->6)]-beta-D-glucopyranoside (6), (25R, 26R)-17alpha-hydroxy-26-methoxyspirost-5-ene-3beta-yl-O-alpha-L-rhamnopyranosyl-(1-->2)-[beta-D-glucopyra nosyl-(1-->6)]-beta-D-glucopyranoside (7), daucosterol (8), adenoside (9), berberine (10).
CONCLUSIONS:
All compounds except 1 and 3 were isolated from this species for the first time, and berberine was first reported in genus Lilium.