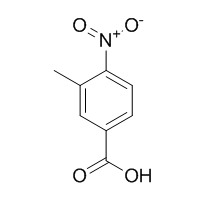
3-Methyl-4-nitrobenzoic acid
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Antimicrob Agents Chemother.2024, e0031424.
Turk J Med Sci.2023 53: 1312-1320.
Sci Rep. 2024, 14(1):70.
Foods.2022, 11(12):1773.
Natural Product Communications2020, doi: 10.1177.
Pharmaceuticals (Basel).2024, 17(8):988.
ACS Synth Biol.2022, doi: 10.1021.
Viruses2023, 15(6), 1377
Antibiotics.2022, 11(4), 510.
Kaohsiung J Med Sci.2023, 10.1002/kjm2.12764
Related and Featured Products
《Chinese Journal of Applied Chemistry》 2005-12
Synthesis of 3-Methyl-4-nitrobenzoic Acid via Catalytic Oxidation with Molecular Oxygen[Reference:
WebLink]
METHODS AND RESULTS:
3-Methyl-4-nitrobenzoic acid was synthesized via oxidation of 2,4-(dimethylnitrobenzene) with mole-(cular) oxygen catalyzed by cobalt acetate in acetic acid in the presence of an initiator.It was found that the yield of 3-Methyl-4-nitrobenzoic acid increased remarkably with the addition of sodium bromide as a co-(catalyst).The reaction was carried out at 130 ℃ for 8 h at 0.8 MPa of oxygen in the presence of 0.017 mol of cobalt acetate,(0.017 mol) of sodium bromide,and 0.30 mol of 2-butanone with 1.00 mole of 2,4-dimethylnitrobenzene.(Under) these conditions 99% of the reactant converted with a selectivity of 52% to 3-methyl-4-(nitrobenzoic) acid,and the yield of 3methyl-4-(nitrobenzoic) acid was 51%.The reaction mechanism and the function of(sodium) bromide were discussed.
CONCLUSIONS:
According to the fact that the reaction could not proceed without the presence of an initiator,it was proposed that the reaction took place via a free radical mechanism,and(sodium) bromide reacted with Co(Ⅲ) to generate bromine radicals,which promoted the formation of benzyl radicals.