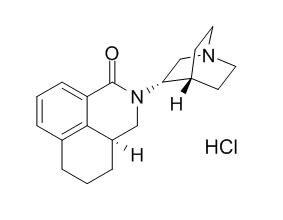
Palonosetron hydrochloride
Palonosetron hydrochloride is the only serotonin receptor antagonist approved for prevention of delayed chemotherapy-induced nausea and vomiting (CINV) caused by moderate emetogenic chemotherapy (MEC).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Mol Cell.2017, 68(4):673-685
Int J Cosmet Sci.2022, doi:10.1111/ics.12827.
Applied Biological Chemistry2024, 67:66.
Pharmaceuticals (Basel).2021, 14(7):633.
J Int Med Res.2021, 49(7):3000605211032849.
Microchemical Journal2024, 200:110475
Molecules.2019, 24(9):E1719
J Korean Soc Food Sci Nutr2023, 52(7): 750-757
Applied Biological Chemistry2023, 66:8
Appl. Sci.2020, 10(8),2804
Related and Featured Products
Expert Opin Pharmacother. 2013 Apr;14(5):629-41.
An update on palonosetron hydrochloride for the treatment of radio/chemotherapy-induced nausea and vomiting.[Pubmed:
23414148]
Nausea and vomiting are well recognized in different clinical situations, suggesting that no single mechanism is likely to be responsible for their production.
METHODS AND RESULTS:
Chemotherapy-induced nausea and vomiting (CINV) can have a negative impact on quality of life and this may lead to a refusal of curative therapy or to a decline in palliative benefits offered by cytotoxic treatment. Palonosetron hydrochloride is a new agent in the class of 5-HT3 receptor antagonists (5-HT3RAs), and differs from the other agents by its higher receptor-binding affinity and longer half-life. These pharmacological properties have resulted in improved antiemetic activity in clinical trials, particularly in the treatment of delayed CINV following moderate emetogenic chemotherapy (MEC). A systematic review of the medical literature was completed to inform this update. MEDLINE, the Cochrane Collaboration Library and meeting materials from ASCO and MASCC were all searched.
CONCLUSIONS:
Palonosetron hydrochloride was the only serotonin receptor antagonist approved for prevention of delayed CINV caused by MEC and its use was incorporated in guideline recommendations. To date, several treatment settings such as multiple day chemotherapy require further studies to improve emesis related to therapy.
Exp Ther Med. 2013 May;5(5):1418-1426.
The effect of palonosetron hydrochloride in the prevention of chemotherapy-induced moderate and severe nausea and vomiting.[Pubmed:
23737892]
The current study aimed to evaluate the efficacy and safety of Palonosetron hydrochloride injection for preventing chemotherapy-induced moderate and severe nausea and vomiting.
METHODS AND RESULTS:
A multi-centered, randomly stratified, double-blind, double-dummy, parallel-group and positive-controlled trial was performed. A total of 240 patients who underwent chemotherapy treatment which induced moderate or severe vomiting were divided into the experimental and control groups. Half an hour before chemotherapy, the experimental group received a 0.25-mg Palonosetron hydrochloride injection, whereas the control group received a 3-mg granisetron injection. The acute vomiting complete remission rate (CRR) of the experimental group was not significantly different compared with that of the control group (P=0.35). The delayed vomiting CRR of the experimental group was significantly higher compared with that of the control group (P=0.002). No difference in full course vomiting CRR, vomiting control time, treatment failure time or acute nausea CRR was identified between the two groups. No significant differences in adverse events were observed between the experimental group and the control group. No significant differences in adverse reactions occurred between the experimental group and the control group (12.50%).
CONCLUSIONS:
Palonosetron hydrochloride injection had a better effect on delayed vomiting CRR than granisetron hydrochloride injection. The two injections exhibited similar effects on acute vomiting CRR, full course vomiting CRR, vomiting control time, treatment failure time (days), acute nausea CRR and adverse events.
J Chromatogr A. 2014 May 16;1342:86-91.
Effect of low concentration sodium dodecyl sulfate on the electromigration of palonosetron hydrochloride stereoisomers in micellar electrokinetic chromatography.[Pubmed:
24709591]
METHODS AND RESULTS:
The effect of low concentrations of sodium dodecyl sulfate (SDS) on the separation of Palonosetron hydrochloride (PALO) stereoisomers by micellar electrokinetic chromatography (MEKC) has been investigated. It was found that the addition of SDS prolongs the migration time and the migration order of four stereoisomers changes regularly with the SDS concentration. Good separations for all the four stereoisomers were achieved at appropriate SDS concentration. The effect of SDS on the electromigration (mobilities) of Palonosetron hydrochloride stereoisomers has been studied, in order to explain its effect on the separation by MEKC. It was found that low concentrations of SDS added into the separation media forms negatively charged complexes with Palonosetron hydrochloride stereoisomers and hence reverses their electromigration direction. Furthermore, the migration order between two enantiomeric pairs is also reversed because the enantiomeric pair with a bigger positive mobility than that of another pair turns to have a bigger negative mobility when bound with SDS.
CONCLUSIONS:
Based on these results, the effect of SDS on the MEKC separation of Palonosetron hydrochloride stereoisomers was elucidated reasonably. The performance of the developed chiral MEKC method was validated by the analysis of a real sample.