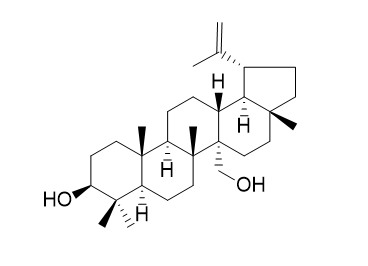
Obtusalin
Obtusalin has some antibacterial activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Cosmet Sci.2022, doi:10.1111/ics.12827.
Foods.2020, 9(10):1348.
Kyung Hee University2024, rs-3888374
Plants (Basel).2023, 12(1):163.
Plos One.2020, 10.1371
Molecules.2020, 25(18),4089.
Agronomy2023, 13(6), 1435.
Journal of Functional Foods2022, 99: 105331.
Neuropharmacology2019, 151437
Bioorg Chem.2024, 152:107720.
Related and Featured Products
Chin J Nat Med . 2015 Apr;13(4):307-310
Antibacterial constituents from Melodinus suaveolens[Pubmed:
25908630]
To investigate the non-alkaloidal chemical constituents of the stems and leaves of Melodinus suaveolens and their antibacterial activities. Compounds were isolated and purified by repeated silica gel, Sephadex LH-20, RP18, and preparative HPLC. Their structures were elucidated by comparison with published spectroscopic data, as well as on the basis of extensive spectroscopic analysis. The antibacterial screening assays were performed by the dilution method. Fourteen compounds were isolated, and identified as lycopersene (1), betulinic aldehyde (2), 3β-acetoxy-22,23,24,25,26,27-hexanordammaran-20-one (3), 3a-acetyl-2, 3, 5-trimethyl-7a-hydroxy-5-(4,8,12-trimethyl-tridecanyl)-1,3a,5,6,7,7a-hexahydro-4-oxainden-1-one (4), 3β-hydroxy-28-norlup-20(29)-ene-17β-hydroperoxide (5), 3β-hydroxy-28-norlup-20(29)-ene-17α-hydroperoxide (6), β-sitosterol (7), 28-nor-urs-12-ene-3β, 17β-diol (8), α-amyrin (9), ergosta-4,6,8(14),22-tetraen-3-one (10), 3β-hydroxy-urs-11-en-28,13β-olide (11), betulin (12), Obtusalin (13), and ursolic acid (14). Among the isolates, compounds 1, 2, 6, 8, 10, and 14 showed potent antibacterial activities against the four bacteria. This is the first report of the antibacterial activity of the constituents of Melodinus suaveolens.
Chin J Nat Med . 2015 Apr;13(4):307-310.
Antibacterial constituents from Melodinus suaveolens[Pubmed:
25908630]
To investigate the non-alkaloidal chemical constituents of the stems and leaves of Melodinus suaveolens and their antibacterial activities. Compounds were isolated and purified by repeated silica gel, Sephadex LH-20, RP18, and preparative HPLC. Their structures were elucidated by comparison with published spectroscopic data, as well as on the basis of extensive spectroscopic analysis. The antibacterial screening assays were performed by the dilution method. Fourteen compounds were isolated, and identified as lycopersene (1), betulinic aldehyde (2), 3β-acetoxy-22,23,24,25,26,27-hexanordammaran-20-one (3), 3a-acetyl-2, 3, 5-trimethyl-7a-hydroxy-5-(4,8,12-trimethyl-tridecanyl)-1,3a,5,6,7,7a-hexahydro-4-oxainden-1-one (4), 3β-hydroxy-28-norlup-20(29)-ene-17β-hydroperoxide (5), 3β-hydroxy-28-norlup-20(29)-ene-17α-hydroperoxide (6), β-sitosterol (7), 28-nor-urs-12-ene-3β, 17β-diol (8), α-amyrin (9), ergosta-4,6,8(14),22-tetraen-3-one (10), 3β-hydroxy-urs-11-en-28,13β-olide (11), betulin (12), Obtusalin (13), and ursolic acid (14). Among the isolates, compounds 1, 2, 6, 8, 10, and 14 showed potent antibacterial activities against the four bacteria. This is the first report of the antibacterial activity of the constituents of Melodinus suaveolens.
Zhongguo Zhong Yao Za Zhi . 2020 Aug;45(15):3689-3693.
[A new macrocyclic phenolic glycoside from Sorghum vulgare root][Pubmed:
32893559]
Eleven compounds were isolated and purified from Sorghum vulgare root extract, through column chromatography over silica gel, MCI gel, and preparative HPLC. Their structures were established by MS, 1 D NMR and 2 D NMR data as sorgholide A(1), β-sitosterol(2), stigmastero(3), daucosterol(4), 4-methoxycinnamic acid(5), taxiphyllin(6), chlorogenic acid(7), p-hydroxybenzaldehyde(8), succini acid(9), trans-p-hydroxycinnamic acid(10), Obtusalin(11). Compounds 4,5 and 9-11 were reported from this species for the first time, and compound 1 is the first 24 ring dimeric double lactonol glycoside formed by reverse polymerization of p-hydroxyphenylacetate glucoside, named sorgholide A.
Nat Prod Res . 2020 Dec;34(23):3313-3319.
A new pentacyclic triterpenoid from the leaves of Rhododendron dauricum L. with inhibition of NO production in LPS-induced RAW 264.7 cells[Pubmed:
30810367]
A new pentacyclic triterpenoid, 3-oxo-urs-11,13(18)-dien-28-oic acid (1), along with twelve known triterpenoids, α-amyrin (2), 19α-hydroxy-α-amyrin (3), triptohypol E (4), uvaol (5), 2α,3α-dihydroxyurs-11-en-13β,28-olide (6), 3β-hydroxyurs-11-en-13β,28-olide (7), ursolic acid (8), asiatic acid (9), oleanolic acid (10), aegiceradienol (11), Obtusalin (12) and betulinic acid (13) were isolated from the leaves of Rhododendron dauricum L. Their structures were established from spectroscopic data and comparison with reported values. Among them, compounds 3, 4, 6, 7 and 11 were isolated from the Ericaceae family for the first time. Compounds 2, 5, 9, 12 and 13 were obtained from R. dauricum for the first time. Additionally, compounds 6, 10 and 11 significantly inhibited the levels of NO in LPS-stimulated RAW 264.7 cells at 3 μM.
Zhongguo Zhong Yao Za Zhi . 2010 Feb;35(3):315-322.
[Terpenoids of Heteroplexis micocephala and their bioactivities][Pubmed:
20422996]
Objective: To investigate the chemical constituents of Heteroplexis nicocephala.
Method: The constituents were isolated by using a combination of various chromatographic techniques including column chromatography over silica gel, Pharmadex LH-20, and C-18, as well as reversed-phase HPLC. Structures of the isolates were identified by spectroscopic data analysis. In vitro cytotoxic, neuroprotective, and anti-inflammatory activities were screened by using cell-based models.
Result: Seventeen terpenoids were isolated. The structures were identified as two monoterpenoids: (-)-bomyl ferulate(1) and loliolide(2). Seven sesquiterpenoids: 1beta-hydroxy-alpha-cyperone(3) , alpha-rotunol(4), 10alpha-hydroxycadin-4-en-15-al (5), 1-epi-10beta-hydroxycadin-4-en-15-al(6), 10alpha-hydroxyisodauc-3-en-15-al(7), germacrene B(8), and mandassidione(9). Five diterpenoids: 12-epi-bacchotricuneatin A(10), 1-hydroxy-12-epi-bacchotricuneatin A(11), cleroinermin(12), desoxyarticulin(13), and anhydroolearin(14). And three triterpenoids: friedelin (15), ursolic acid(16), and Obtusalin(17). In the in vitro assays, 1 showed selective cytotoxic activity against BGC-823, with an IC50 value of 8.00 x 10(-5) mol x L(-1). At a concentration of 1 x 10(-5) mol x L(-1), 12 showed neuroprotective activity against MPP+ induced PC12-syn cell damage, with a relative cell proliferation rate of 104.32% (P < 0.001). 2 exhibited inhibitory activity against the release of beta-glucuronidase from the polymorphous nuclear leukocytes induced by PAF, with an inhibitory rate of 52.7% (P < 0.05) at the same concentration.
Conclusion: Compounds 1-17 were obtained from the genus Heteroplexis for the first time. 1 showed selective cytotoxic activity against human gastric cancer cell lines (BGC-823), 12 and 2 showed potent neuroprotective and anti-inflammatory activities, respectively.