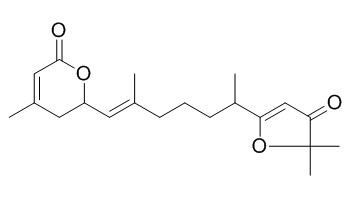
Nemoralisin
Nemoralisin exhibits weak cytotoxicities (IC50>10 uM) against HepG2, AGS, MCF-7, and A-549 cancer cell lines.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2021, 22(16):8641.
Industrial Crops and Products2024, 219:119123
Molecules.2021, 26(4):816.
Biofactors.2018, 44(2):168-179
Cells.2021, 10(10):2633.
Sci Rep.2018, 8(1):12970
Trop J Nat Prod Res.2019, 3(1):6-9
Scientific World Journal.2014, 2014:654193
Front Microbiol.2024, 15:1429027.
Front Pharmacol.2025, 16:1611342.
Related and Featured Products
Fitoterapia. 2014 Jan;92:100-4.
Chemical constituents from Aphanamixis grandifolia.[Pubmed:
24188860]
METHODS AND RESULTS:
Four new terpenoids, Nemoralisins D-G (1-4), were isolated from the leaves and stems of Aphanamixis grandifolia, along with two known diterpenoids, Nemoralisin C and Nemoralisin. Among them, compound 1 is the first example of norsesquiterpenoid with δ-lactone moiety, and Nemoralisins E-G (2-4), are a class of acyclic diterpenoids, which are structurally related Nemoralisin C and Nemoralisin. These structures were established on the basis of spectroscopic methods and the absolute configuration of 1 was determined by comparison of quantum chemical TDDFT calculated and experimental ECD spectra.
CONCLUSIONS:
Nemoralisins D-G (1-4) were tested for their cytotoxicities on HL-60, SMMC-7721, A-549, MCF-7, and SW480 human tumor cell lines (IC50>40 μM), as well as the antimicrobial activities on Staphylococcus aureus, Pseudomonas aeruginosa, MRSA92(#) and MRSA98(#) (MIC>50 μg/mL).
Chem Pharm Bull (Tokyo). 2014;62(5):494-8.
Aphanamixins A-F, acyclic diterpenoids from the stem bark of Aphanamixis polystachya.[Pubmed:
24789934]
METHODS AND RESULTS:
Six new acyclic diterpenoids named Aphanamixins A-F (1-6), together with two known compounds of Nemoralisin and Nemoralisin C, were isolated from the stem bark of Aphanamixis polystachya (WALL) J. N. BARKER. Their structures were established through a comprehensive analysis of NMR spectroscopic data and high resolution mass spectrometric data. The absolute configurations of carbon stereocenters were determined by means of auxiliary chiral α-methoxy-α-(trifluoromethyl)phenylacetic acid (MTPA) derivatives and circular dichroism (CD), respectively.
CONCLUSIONS:
All the new isolates were tested for their antiproliferative activity against HepG2, AGS, MCF-7, and A-549 cancer cell lines and they exhibited weak cytotoxicities (IC50>10 µM). Moreover, we highlighted that the six new diterpenoids characterized by acyclic skeleton was rarely seen in nature.