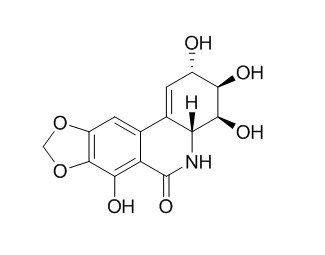
Narciclasine
The alkaloid Narciclasine is the bioactive compound responsible for the anti-inflammatory action of HCE. Narciclasine displays marked proapoptotic and cytotoxic activity, as does pancratistatin, and significant in vivo anticancer effects in various experimental models, but it is also associated with severe toxic side effects. Narciclasine acts on the auxin signaling pathway upstream of TIR1, which modulates Aux/IAA protein degradation, and thereby affects the auxin-mediated responses in Arabidopsis roots, inhibits the root hair development of lettuce seedlings.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2023, 28(9):3685.
Int J Mol Sci.2024, 25(18):9909.
Int J Cosmet Sci.2019, 41(1):12-20
Journal of Ginseng Research2021, 25 November
Antioxidants (Basel).2021, 10(9):1435.
Korean J. Agricultural Science2024, 51(3).
Anal Bioanal Chem. 2025, 417(17):3879-3892.
Molecules.2020, 25(18),4089.
J Pain Res.2022, 15:3469-3478.
Neuropharmacology.2018, 131:68-82
Related and Featured Products
Protoplasma. 2014 Sep;251(5):1113-24.
Inhibition of root growth by narciclasine is caused by DNA damage-induced cell cycle arrest in lettuce seedlings.[Pubmed:
24482192]
Narciclasine (NCS) is an Amaryllidaceae alkaloid isolated from Narcissus tazetta bulbs.
METHODS AND RESULTS:
Its phytotoxic effects on plant growth were examined in lettuce (Lactuca sativa L.) seedlings. Results showed that high concentrations (0.5-5 μM) of Narciclasine restricted the growth of lettuce roots in a dose-dependent manner. In Narciclasine-treated lettuce seedlings, the following changes were detected: reduction of mitotic cells and cell elongation in the mature region, inhibition of proliferation of meristematic cells, and cell cycle. Moreover, comet assay and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay indicated that higher levels Narciclasine (0.5-5 μM) induced DNA damage in root cells of lettuce. The decrease in meristematic cells and increase in DNA damage signals in lettuce roots in responses to Narciclasine are in a dose-dependent manner. Narciclasine-induced reactive oxygen species accumulation may explain an increase in DNA damage in lettuce roots.
CONCLUSIONS:
Thus, the restraint of root growth is due to cell cycle arrest which is caused by Narciclasine-induced DNA damage. In addition, it was also found that Narciclasine (0.5-5 μM) inhibited the root hair development of lettuce seedlings. Further investigations on the underlying mechanism revealed that both auxin and ethylene signaling pathways are involved in the response of root hairs to Narciclasine.
Planta. 2012 Aug;236(2):597-612.
Narciclasine inhibits the responses of Arabidopsis roots to auxin.[Pubmed:
22476291]
The plant hormone auxin plays a central role in the regulation of plant growth and development, as well as in responses to environmental stimuli. Narciclasine (NCS, an Amaryllidaceae alkaloid) isolated from Narcissus tazetta bulbs has a broad range of inhibitory effects on plants.
METHODS AND RESULTS:
In this study, the role of Narciclasine in responses to auxin in Arabidopsis thaliana roots was investigated. We demonstrated the inhibitory effects of Narciclasine on auxin-inducible lateral root formation, root hair formation, primary root growth, and the expression of primary auxin-inducible genes in Arabidopsis roots using DR5::GUS reporter gene, native auxin promoters (IAA12::GUS, IAA13::GUS), and quantitative reverse transcription PCR analysis. Results also showed that Narciclasine did not affect the expression of cytokinin-inducible ARR5::GUS reporter gene. Narciclasine relieved the auxin-enhanced degradation of the Aux/IAA repressor modulated by the SCFTIR1 ubiquitin-proteasome pathway. In addition, Narciclasine did not alter the auxin-stimulated interaction between IAA7/AXR2 (Aux/IAA proteins) and the F-box protein TIR1 activity of the proteasome.
CONCLUSIONS:
Taken together, these results suggest that Narciclasine acts on the auxin signaling pathway upstream of TIR1, which modulates Aux/IAA protein degradation, and thereby affects the auxin-mediated responses in Arabidopsis roots.
Med Res Rev. 2013 Mar;33(2):439-55.
Narciclasine as well as other Amaryllidaceae isocarbostyrils are promising GTP-ase targeting agents against brain cancers.[Pubmed:
22419031]
The anticancer activity of Amaryllidaceae isocarbostyrils is well documented.
METHODS AND RESULTS:
At pharmacological concentrations, that is, approximately 1 μM in vitro and approximately 10 mg/kg in vivo, Narciclasine displays marked proapoptotic and cytotoxic activity, as does pancratistatin, and significant in vivo anticancer effects in various experimental models, but it is also associated with severe toxic side effects. At physiological doses, that is, approximately 50 nM in vitro and approximately 1 mg/kg in vivo, Narciclasine is not cytotoxic but cytostatic and displays marked anticancer activity in vivo in experimental models of brain cancer (including gliomas and brain metastases), but it is not associated with toxic side effects. The cytostatic activity of Narciclasine involves the impairment of actin cytoskeleton organization by targeting GTPases, including RhoA and the elongation factor eEF1A. We have demonstrated that chronic treatments of Narciclasine (1 mg/kg) significantly increased the survival of immunodeficient mice orthotopically xenografted with highly invasive human glioblastomas and apoptosis-resistant brain metastases, including melanoma- and non-small-cell-lung cancer- (NSCLC) related brain metastases.
CONCLUSIONS:
Thus, Narciclasine is a potentially promising agent for the treatment of primary brain cancers and various brain metastases. To date, efforts to develop synthetic analogs with anticancer properties superior to those of Narciclasine have failed; thus, research efforts are now focused on Narciclasine prodrugs.
J Cell Mol Med. 2015 May;19(5):1021-32.
Haemanthus coccineus extract and its main bioactive component narciclasine display profound anti-inflammatory activities in vitro and in vivo.[Pubmed:
25754537]
Haemanthus coccineus extracts (HCE) have traditionally been used to treat a variety of diseases, like febrile colds or asthma. Since new therapeutic options against inflammatory processes are still urgently needed, we aimed to pharmacologically characterise the anti-inflammatory potential of HCEin vitro and in vivo and to identify the underlying bioactive component(s).
METHODS AND RESULTS:
The action of HCE on oedema formation and leucocyte infiltration were analysed in two murine models of inflammation (dermal oedema induced by arachidonic acid and croton oil; kidney injury caused by unilateral ureteral obstruction). The interaction of leucocytes with endothelial cells (ECs) as well as the activation parameters of these two cell types were analysed. Moreover, the nuclear factor κB (NFκB) pathway was investigated in detail in ECs. Using different fractions of HCE, the bioactive principle was identified. In vivo, HCE (450 mg/kg orally or 2 mg/kg intraperitoneally) inhibited oedema formation, leucocyte infiltration and cytokine synthesis. In vitro, HCE (100-300 ng/ml) blocked leucocyte-EC interaction as well as the activation of isolated leucocytes (cytokine synthesis and proliferation) and of primary ECs (adhesion molecule expression). HCE suppressed NFκB-dependent gene transcription in the endothelium, but did not interfere with the NFκB activation cascade (IκB degradation, p65 nuclear translocation and NFκB DNA-binding activity). The alkaloid Narciclasine was elucidated as the bioactive compound responsible for the anti-inflammatory action of HCE.
CONCLUSIONS:
Our study highlights HCE and its main alkaloid Narciclasine as novel interesting approach for the treatment of inflammation-related disorders.
Bioorg Med Chem Lett. 2014 Sep 1;24(17):4236-8.
Synthesis and biological evaluation of unnatural derivatives of narciclasine: 7-aza-nornarciclasine and its N-oxide.[Pubmed:
25108300]
METHODS AND RESULTS:
Several unnatural derivatives of Narciclasine were prepared in which the C-7 carbon was replaced with nitrogen. The 7-aza derivative and its N-oxide were prepared by the coupling of iodopicolinic acid with a conduramine unit derived chemoenzymatically from bromobenzene.
CONCLUSIONS:
Intramolecular Heck reaction was used to construct the isocarbostyryl ring system. The compounds were submitted to biological screening against cancer cell lines. Full experimental and spectra data are provided for all new compounds.